1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione CAS#: 1150560-54-5; ChemWhat Code: 1052789
Identification
| Product Name | 1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione |
| IUPAC Name | 1-[[2-fluoro-6-(trifluoromethyl)phenyl]methyl]-5-iodo-6-methylpyrimidine-2,4-dione |
| Molecular Structure | ![Structure of 1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione CAS 1150560-54-5](https://www.chemwhat.com/wp-content/uploads/2018/06/Structure-of-1-2-fluoro-6-trifluoromethylbenzyl-5-iodo-6-methylpyrimidine-241H3H-dione-CAS-1150560-54-5.png) |
| CAS Registry Number | 1150560-54-5 |
| EINECS Number | No data available |
| MDL Number | MFCD19441362 |
| Beilstein Registry Number | No data available |
| Synonyms | 1-{[2-fluoro-6-(trifluoromethyl)phenyl]methyl}-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione, 1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methyl-3H-pyrimidine-2,4-dione, 1-(2-fluoro-6-trifluoromethyl-benzyl)-5-iodo-6-methyl-1H-pyrimidine-2,4-dione;CAS Number: 1150560-54-5 |
| Molecular Formula | C13H9F4IN2O2 |
| Molecular Weight | 428.121 |
| InChI | InChI=1S/C13H9F4IN2O2/c1-6-10(18)11(21)19-12(22)20(6)5-7-8(13(15,16)17)3-2-4-9(7)14/h2-4H,5H2,1H3,(H,19,21,22) |
| InChI Key | TZBUKGISILNJCR-UHFFFAOYSA-N |
| Canonical SMILES | CC1=C(C(=O)NC(=O)N1CC2=C(C=CC=C2F)C(F)(F)F)I |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN110437159 | Preparation method of gonadotropin releasing hormone antagonist intermediate and antagonist sodium (by machine translation) | 2019 |
| WO2009/62087 | PROCESSES FOR THE PREPARATION OF URACIL DERIVATIVES | 2009 |
Physical Data
| Appearance | White or off-white powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
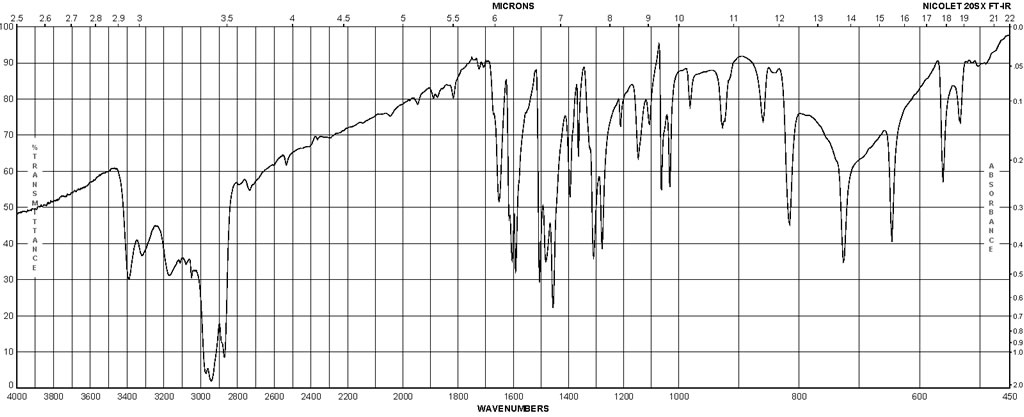
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz | Original Text (NMR Spectroscopy) |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 300 | NMR (400 MHz, DMSO-d6) d 11.75 (s, 1H), 77.67-7.61 (m, 1H), 7.59-7.50 (m, 2H), 5.39 (s,1H), 2.56 (s, 3H). |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands | potassium bromide | 27 |
| Spectrum | CCl4 | 14.85 – 54.85 |
| Description (Mass Spectrometry) | Comment (Mass Spectrometry) |
| ESI (Electrospray ionisation), LCMS (Liquid chromatography mass spectrometry) | Molecular peak |
Route of Synthesis (ROS)
![Route of Synthesis (ROS) of 1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione CAS 1150560-54-5](https://www.chemwhat.com/wp-content/uploads/2018/06/Route-of-Synthesis-ROS-of-1-2-fluoro-6-trifluoromethylbenzyl-5-iodo-6-methylpyrimidine-241H3H-dione-CAS-1150560-54-5.png)
| Conditions | Yield |
| With Iodine monochloride In methanol at 50℃; for 6h; Experimental Procedure To a 250 mL three-neck round bottom flask was charged l-(2-fluoro-6- trifluoromethyl-benzyl)-6-methyl-lH-pyrimidine-2,4-dione Ia (25.0 g) and methanol (250 mL). Iodine monochloride (30.9 g) was charged over 2.5 min. The mixture was heated at 50 0C for 3 hours. After cooling to ambient temperature, the mixture was filtered. The cake was re-slurried in methanol (250 mL) and heated to 50 C for 3 hours. After cooling to ambient temperature, the mixture was filtered and the filter cake was washed with methanol (50 mL). The off-white solid was dried in a vacuum oven at 50 C to provide l-(2-fluoro-6-trifluoromethyl-benzyl)-5-iodo-6-methyl-lH-pyrimidine-2,4- dione Ib (32.5 g, 90% yield). LCMS (ESI) m/z 429.3 (MH+). The material may be re- slurried in MeOH as needed. | 90% |
| With N-iodo-succinimide; acetic acid at 50℃; for 1h; Experimental Procedure To a 50 mL round botom flask was added AcOH (20 mL), compound IX (2 g) and N-Iodosuccinimide (1.5 g). The reaction mixture was heated to 50 °C for lh. After completion of the reaction, LLO (10 mL) was added to the reaction mixture. The resulting suspension was filtered. The cake was washed with MeOH (4 mL). The solid was slurried with MeOH (20 mL) to give the product as a white solid (2.3 g, 82% yield, 96.1% purity). NMR (400 MHz, DMSO-d6) d 11.75 (s, 1H), 77.67-7.61 (m, 1H), 7.59-7.50 (m, 2H), 5.39 (s, 1H), 2.56 (s, 3H). | 82% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Warning |
| GHS Hazard Statements | H302 (33.33%): Harmful if swallowed [Warning Acute toxicity, oral] H312 (33.33%): Harmful in contact with skin [Warning Acute toxicity, dermal] H315 (66.67%): Causes skin irritation [Warning Skin corrosion/irritation] H320 (66.67%): Causes eye irritation [Warning Serious eye damage/eye irritation] H332 (33.33%): Harmful if inhaled [Warning Acute toxicity, inhalation] H361 (66.67%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P201, P202, P261, P264, P270, P271, P280, P281, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P308+P313, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | No data available |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 428.125 |
| logP | 3.322 |
| HBA | 4 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 49.41 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Use Pattern |
| 1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione CAS#: 1150560-54-5 is usually used as an intermediate of API, Elagolix |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |