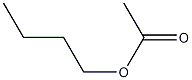
1-Butyl acetate CAS#: 123-86-4; ChemWhat Code: 114276
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2020/28214 | ELECTROLYTE SOLUTION, ELECTROCHEMICAL DEVICE, LITHIUM ION SECONDARY BATTERY AND MODULE | 2020 |
| CN107325065 | Preparation method of 2,5-furandicarboxylic acid | 2017 |
| CN105237342 | Method for preparing alcohol through catalytic hydrogenation reduction of carboxylate | 2016 |
| WO2015/127372 | PROCESS FOR MAKING ESTERS OF 2-ACETOXYALKANOIC ACIDS USING AN ALPHA-HYDROXYALKANOIC ACID ESTER AND AN ACETATE ESTER AS STARTING MATERIALS | 2015 |
Physical Data
| Appearance | Transparent liquid.no visible impurities |
| Butyl alcohol,%(m/m) | ≤0.13 |
| Acidity(as acetic acid%)(m/m) | ≤0.004 |
| Moisture,%(m/m) | ≤0.2 |
| Melting Point, °C |
| -78.85 |
| 120 – 126 |
| -73.5 |
| -73.3 |
| -77.9 |
| -76.8 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 126 | |
| 125.8 | |
| 125 | |
| 118 | |
| 125.95 | 759.826 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 29.99 | |
| 44.99 | |
| 39.99 | |
| 34.99 | |
| 24.99 | |
| 0.85537 | 44.99 |
| Description (Association (MCS)) | Temperature (Association (MCS)), °C |
| Adsorption | |
| Desorption | |
| Adsorption | 80 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | ||
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 25 | 75 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands, Spectrum | |
| Bands, Spectrum | potassium bromide |
| Bands | |
| Bands | KBr |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) |
| Spectrum | decane | 357.14 – 200 nm |
| Absorption maxima | ||
| Spectrum | 900 – 2400 nm | |
| Spectrum | 700 – 2500 nm |
Route of Synthesis (ROS)
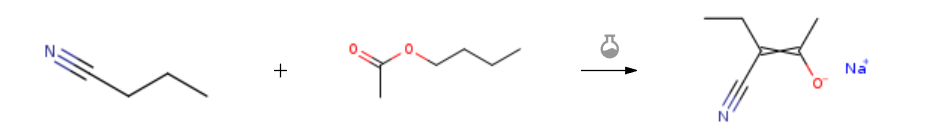
Route of Synthesis (ROS) of 1-Butyl acetate CAS 123-86-4
| Conditions | Yield |
| With sodiu mmethylate In xylene at 150℃; under 2175.22 Torr; for 2h; Experimental Procedure Example 10 Synthesis of sodium salt of 3-cyano-2-pentanone In an autoclave made of glass having an inner volume of 300 ml and equipped with a stirring device, a thermometer and a pressure gauge were charged 30.2 g (0.26 mol) of n-butyl acetate, 41.7 g-(0.60 mol) of butyronitrile, 10.8 g (0.20 mol) of sodium methoxide and 83 ml of xylene, and the mixture was reacted at 150° C. under spontaneous pressure (0.29 MPa (gauge pressure)) in a closed reaction vessel for 2 hours under nitrogen atmosphere.After completion of the reaction, the mixture was cooled to room temperature, and precipitated products were collected by filtration and dried to give 23.4 g of a sodium salt of 3-cyano-2-pentanone (isolation yield: 87.9%) as colorless powder. Physical property of sodium salt of 3-cyano-2-pentanone was as follows. 1H-NMR (DMSO-d6, 6 (ppm)); 0.83 (3H, t), 1.73 (3H, s), 1.92 (2H, q) | 87.9% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H226: Flammable liquid and vapor [Warning Flammable liquids] H336: May cause drowsiness or dizziness [Warning Specific target organ toxicity, single exposure; Narcotic effects] |
| Precautionary Statement Codes | P210, P233, P240, P241, P242, P243, P261, P271, P280, P303+P361+P353, P304+P340, P319, P370+P378, P403+P233, P403+P235, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
No data available
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 116.16 |
| logP | 1.591 |
| HBA | 2 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 26.3 |
| Rotatable Bond (RotB) | 4 |
| Matching Veber Rules | 2 |
| Quantitative Results | ||
| 1 of 535 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | PROCESS FOR PRODUCING HYDROGENATED ESTER, HYDROGENATION CATALYST FOR USE THEREIN, AND PROCESS FOR PRODUCING THE CATALYST | |
| 2 of 535 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Anti-allergic composition containing zwitterionic bicyclic compounds | |
| 3 of 535 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Novel farnesyl protein transferase inhibitors as antitumor agents | |
| 4 of 535 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | TETRACYCLINE COMPOUNDS HAVING TARGET THERAPEUTIC ACTIVITIES | |
| 5 of 535 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Aqueous dispersion of nanocapsules with an oily core |
| Use Pattern |
| BUTYL ACETATE is used as solvent in coating,lacquer, printing ink, adhesive, leatheroid, nitrocellulose, etc. It is the solvent of some cosmetics, acting as medium boiling solvent of nail polishes to dissolve the epithelium forming agents, like nitrocellulose, acrylate and alkyd resins. It also can be used to prepare the remover of nail polishes. It is often mixed with ethyl acetate while in use. It is also applied to prepare perfume. It appea「s in the recipes of apricot, banana, pear and pineapple essences.In petroleum refining and pharmaceutical industry, it is used as extractant, especially the extractant of some antibiotics. And BUTYL ACETATE is an azeotrope former with good ab小ty to carry water. It is often used to condense some weak solution to reduce energy consumption. BUTYL ACETATE also can be used as analytical reagent verify thalium, stannum and tungsten, and determine molybdenum and rhenium. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |