1-Pyrenylboronic acid CAS#: 164461-18-1; ChemWhat Code: 1411641
Identification
| Product Name | 1-Pyrenylboronic acid |
| IUPAC Name | pyren-1-ylboronic acid |
| Molecular Structure | 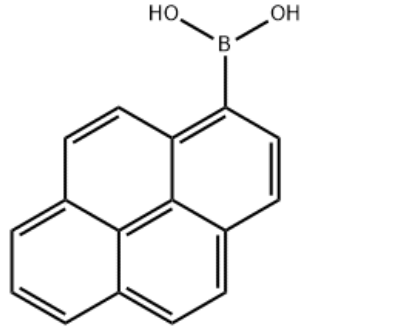 |
| CAS Registry Number | 164461-18-1 |
| MDL Number | MFCD00006400 |
| Synonyms | 1-Pyrenylboronic acid Pyren-1-ylboronic Acid 164461-18-1 1-Pyreneboronic acid Pyrene-1-boronic acid Boronic acid, 1-pyrenyl- 1-pyrenyl boronic acid (pyren-1-yl)boronic acid Boronic acid, B-1-pyrenyl- MFCD04974062 1-Pyreneboronic acid (contains varying amounts of Anhydride) C16H11BO2 pyreneboronic acid pyren-1-boronic acid pyren-1-ylboronicacid SCHEMBL224652 YSSJ01086 DTXSID60408164 BBL103871 Pyrene-1-boronic acid, >=95.0% STL557681 AKOS015840457 AB21450 CS-W010239 FD14070 GS-6482 OL10090 AM808106 SY022635 FT-0653019 P1625 pyren-1-ylboronic acid;1-Pyreneboronic Acid A810568 J-010142 J-524083 1-Pyreneboronic Acid, (contains varying amounts of Anhydride) |
| Molecular Formula | C16H11BO2 |
| Molecular Weight | 246.1 |
| InChI | InChI=1S/C16H11BO2/c18-17(19)14-9-7-12-5-4-10-2-1-3-11-6-8-13(14)16(12)15(10)11/h1-9,18-19H |
| InChI Key | InChI=1S/C16H11BO2/c18-17(19)14-9-7-12-5-4-10-2-1-3-11-6-8-13(14)16(12)15(10)11/h1-9,18-19H |
| Canonical SMILES | B(C1=C2C=CC3=CC=CC4=C3C2=C(C=C1)C=C4)(O)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN111592540 | Imidazonaphthyridine high-performance photoelectric material and application thereof | 2020 |
| CN109180569 | Pyrene structure-containing compound, and organic luminescent device thereof | 2019 |
| CN107652224 | Compound based on dehydrogenated abietic-acid aromatic ring and preparation method thereof | 2018 |
| CN108822049 | Bipolar compound based on pyrene-triazole derivative and application thereof | 2018 |
| CN108947926 | Bipolar compound based on pyrene-oxadiazole derivative and application of bipolar compound | 2018 |
Physical Data
| Appearance | Yellow powder |
| Melting Point, °C |
| 293 – 297 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Partner (Association (MCS)) |
| Association with compound | aq. buffer, dimethyl sulfoxide | 3,4-dihydroxyphenylethylamine |
| Association with compound | aq. buffer, dimethyl sulfoxide | L-epinephrine |
| Association with compound | aq. buffer, dimethyl sulfoxide | norepinephrine |
| Association with compound | aq. buffer, dimethyl sulfoxide | 7-diethylaminocoumarine-3-aldehyde, L-epinephrine |
| Association with compound | acetonitrile | tetrabutylammonium sulfate |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Spectrum | 11B | CD3OD | ||
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | 300 | |
| Chemical shifts, Spectrum | 13C | dimethylsulfoxide-d6 | 75 | |
| Chemical shifts | 1H | tetrahydrofuran-d8 | 300 | |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 400 | |
| Chemical shifts | 13C | dimethylsulfoxide-d6 | 100 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | KBr |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) |
| Spectrum | water |
| Spectrum | toluene |
Route of Synthesis (ROS)
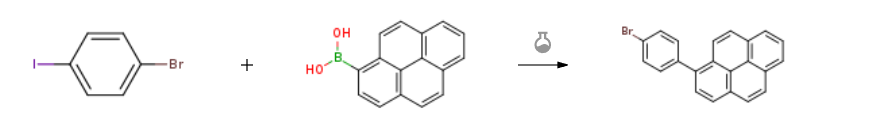
Route of Synthesis (ROS) of 1-Pyrenylboronic acid CAS164461-18-1
| Conditions | Yield |
| With sodium carbonate; tetrakis(triphenylphosphine) palladium(0) In water; toluene at 80℃; for 8h; | 79% |
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In ethanol; water; toluene at 110℃; | 76% |
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In tetrahydrofuran for 4h; Suzuki Coupling; Reflux; | 60% |
| Experimental Procedure Tetrakistriphenylphosphine palladium (2.1 g, 1.83 mmol) and potassium carbonate(75.7 g, 549 mmol) added to 1-indole boric acid(46.0 g, 187 mmol) and p-bromoiodobenzene (51.6 g, 183 mmol)a solution in degassed tetrahydrofuran (500 mL),And the mixture was heated under reflux for 4 hours.Hot filtration, a large amount of solid is obtained, and the solid is dissolved in a solvent.After concentration,Compound d1 was obtained by silica gel column chromatography(39.2 g, yield 60%). |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Cool, dry place with tighten contairier |
| HS Code | |
| Storage | Cool, dry place with tighten contairier |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 246.073 |
| logP | 4.171 |
| HBA | 2 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 40.46 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Use Pattern |
| 1-Pyrenylboronic acid CAS:#164461-18-1 can form coordination complexes with metal ions through the boronic acid group’s coordination ability. These complexes have important applications in catalysis, materials science, and bioanalysis. And 1-Pyreneboronic acid can undergo carbon-carbon bond formation reactions with suitable reactants, such as organic halides or aromatic aldehydes. This reaction is often employed in organic synthesis for carbon-carbon bond connections and the construction of complex organic molecules. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |