1,1′-BIS(DIISOPROPYLPHOSPHINO)FERROCENE CAS#: 97239-80-0; ChemWhat Code: 35929
Identification
| Product Name | 1,1′-BIS(DIISOPROPYLPHOSPHINO)FERROCENE |
| IUPAC Name | DiPPF1 |
| Molecular Structure | 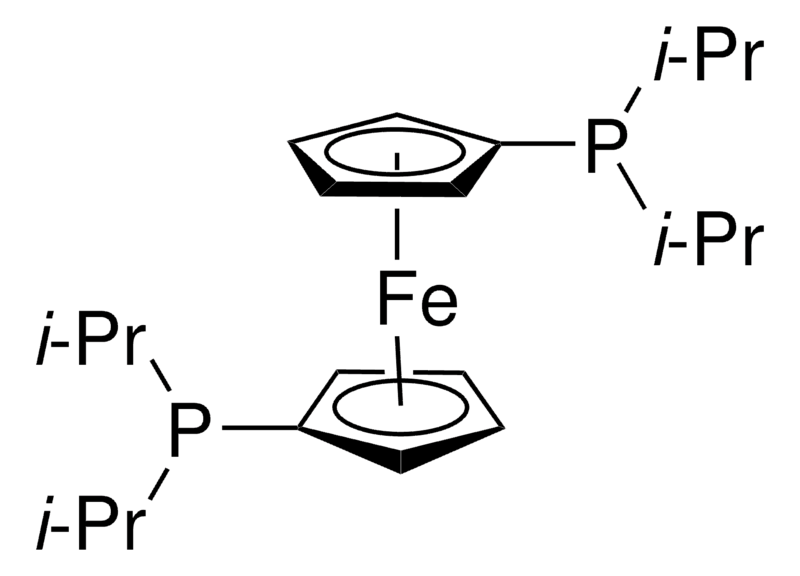 |
| CAS Registry Number | 97239-80-0 |
| EINECS Number | No data available |
| MDL Number | MFCD02684559 |
| Beilstein Registry Number | No data available |
| Synonyms | 1,1′-bis(diisopropylphosphino)ferroceneDiPPF1,1’-bis(diisopropylphosphino)ferrocene1,1′-bis(diisopropylphosphino)ferroceneDiPrPFbis(diisopropylphosphino)ferrocenedipf |
| Molecular Formula | C22H36FeP2 |
| Molecular Weight | 418.323 |
| InChI | InChI=1S/2C11H23P.Fe/c21-9(2)12(10(3)4)11-7-5-6-8-11;/h29-11H,5-8H2,1-4H3; |
| InChI Key | XUUUHBFNNJSDAR-UHFFFAOYSA-N |
| Canonical SMILES | CC(C)P([C]1[CH][CH][CH][CH]1)C(C)C.CC(C)P([C]1[CH][CH][CH][CH]1)C(C)C.[Fe] |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN104861001 | A ferrocene diphosphine ligand preparation method | 2017 |
Physical Data
| Appearance | Orange yellow powder |
| Solubility | Insoluble in water. |
| Flash Point | 230 °F |
| Refractive index | No data available |
| Sensitivity | Air Sensitive & Hygroscopic |
| Melting Point, °C |
| 48 – 51 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C |
| Chemical shifts | 1H | benzene-d6 | 25 |
| Chemical shifts | 13C | benzene-d6 | 25 |
| Chemical shifts | 31P | benzene-d6 | 25 |
| Chemical shifts, Spectrum | 31P | benzene-d6 | 24.84 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | potassium bromide | 488 cm**-1 – 2968 cm**-1 |
| Description (Mass Spectrometry) | Comment (Mass Spectrometry) |
| high resolution mass spectrometry (HRMS), spectrum | |
| Molecular peak, Fragmentation pattern |
| Description (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| dichloromethane | 445 | 110 |
Route of Synthesis (ROS)
| Conditions | Yield |
| Stage #1: acetylacetonato(1,5-cyclooctadiene)rhodium(I) With tetrafluoroboric acid In butanone-2; water for 0.25h; Stage #2: 1,5-cis,cis-cyclooctadiene In water; butanone at 30℃; Stage #3: 1,1′-bis(diisopropylphosphino)ferrocene In water; butanone at 50℃; | 91% |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | Class 6.1; Packaging Group: II; UN Number: 2671 |
| Under the room temperature and away from light | |
| HS Code | 290621 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD 45/kg |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 418.322 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 27.18 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Homogeneous catalyst ligand DiPPF is widely used in homogeneous catalytic reactions, especially in transition metal catalytic reactions, such as palladium-catalyzed cross-coupling reactions (such as Suzuki, Heck, Stille and Sonogashira reactions). Its diphosphine ligand structure can effectively stabilize transition metal centers such as palladium, rhodium and nickel, thereby improving the activity and selectivity of the catalyst. Due to its steric hindrance and electronic effects, DiPPF can significantly improve the yield and efficiency of catalytic reactions and has important applications in the synthesis of complex organic molecules. |
| Drug synthesis In pharmaceutical chemistry, 1,1′-bis(diisopropylphosphino)ferrocene can be used as a catalyst ligand to synthesize biologically active drug molecules. Its catalytic system helps to reduce side reactions and improve the selectivity of target products, which is of great significance in the development of new drugs. |
| Fine chemical synthesis DiPPF is widely used in the synthesis of fine chemicals, fragrances and agricultural chemicals, especially chemical reactions that require high selectivity and high efficiency. Due to its significant promotion of palladium-catalyzed coupling reactions, various complex aromatic and olefin compounds can be synthesized. |
| Materials Science In materials science, DiPPF can be used to synthesize organic electronic materials and functional polymers, such as materials for organic light-emitting diodes (OLEDs) and organic photovoltaic cells (OPVs). Its ligand effect helps to regulate the electronic properties of metal centers, thereby improving the optoelectronic properties of materials. |
| Academic Research In academic research, 1,1′-bis(diisopropylphosphino)ferrocene is widely used as a model ligand to study metal-ligand interactions, catalytic reaction mechanisms, and the development of new catalytic systems. It is used as a tool in coordination chemistry to study different metal complexes and to explore the structure-activity relationship of catalysts. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

