1,12-Dodecanediamine CAS#: 2783-17-7; ChemWhat Code: 1411423
Identification
| Product Name | 1,12-Dodecanediamine ine |
| IUPAC Name | dodecane-1,12-diamine |
| Molecular Structure | |
| CAS Registry Number | 2783-17-7 |
| EINECS Number | 220-489-6 |
| MDL Number | MFCD00008155 |
| Synonyms | 1,12-Diaminododecane 2783-17-7 1,12-DODECANEDIAMINE Dodecane-1,12-diamine Dodecamethylenediamine Dodecyldiamine 1,12-Dodecamethylenediamine 1,12-Diamindodecane 1,12′-Dodecylenediamine 1,12′-Dodecamethylenediamine 1,12-Dodecylenediamine NSC 55050 NSC 59861 N12N 1,12-n-Dodecanediamine 1,12-Diamino-n-dodecane J3LM80W9NT CHEMBL69590 CHEBI:49385 NSC-55050 NSC-59861 EINECS 220-489-6 UNII-J3LM80W9NT BRN 1742765 Dodecylenediamine 1,12diaminododecane dodecamethylene diamine 1,12 diaminododecane 1,12-diamino dodecane 1,12-diamino-dodecane 1,12-dodecane-diamine SCHEMBL27441 4-04-00-01376 (Beilstein Handbook Reference) 1,12-Diaminododecane, 98% DTXSID2044636 NSC55050 NSC59861 ZINC1685531 BBL036612 BDBM50147574 MFCD00008155 STL492207 AKOS015894529 CS-W015599 DB-047282 D0091 FT-0606044 F19630 A819207 Q-200047 Q27121625 |
| Molecular Formula | C12H28N2 |
| Molecular Weight | 200.36 |
| InChI | InChI=1S/C12H28N2/c13-11-9-7-5-3-1-2-4-6-8-10-12-14/h1-14H2 |
| InChI Key | QFTYSVGGYOXFRQ-UHFFFAOYSA-N |
| Canonical SMILES | C(CCCCCCN)CCCCCN |
Physical Data
| Appearance | White flakes |
| Melting Point, °C |
| 70 |
| 68.69 |
| 68.68 |
| 67.38 |
| 42 |
| 66 – 67 |
| 375 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 115 – 125 | 0.60006 |
| 152 | 4 |
| 145 – 148 | 2 |
| 142 – 144 | 5 |
| 135 – 138 | 3 |
| 187 | 16 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C |
| Association with compound | ||
| Association with compound | ||
| Association with compound | ||
| Association with compound | H2O | 22 |
| Further physical properties of the complex | ||
| Stability constant of the complex with … | tetrahydrofuran, H2O | 25 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 300 | |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 300 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1, CD3OD | 500 | |
| Chemical shifts, Spectrum | 13C | chloroform-d1, CD3OD | 126 | |
| Chemical shifts, Spectrum | 1H | water-d2 | 19.94 | 400 |
| Spectrum | 1H | dimethylsulfoxide-d6 | 26.34 | 400 |
| Spectrum | 1H | CD3OD | 26.34 | 400 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands, Spectrum | potassium bromide |
| Bands | neat liquid |
| Spectrum | KBr |
| Description (UV/VIS Spectroscopy) |
| Spectrum |
Route of Synthesis (ROS)
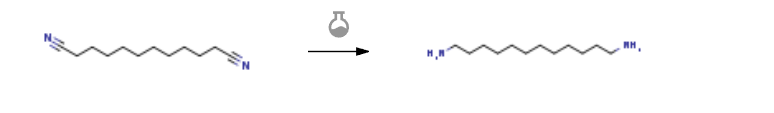
Route of Synthesis (ROS) of 1,12-Dodecanediamine CAS 2783-17-7
| Conditions | Yield |
| With hydrogen; sodium methylate In methanol at 130℃; under 37503.8 Torr; for 5h; | 91.7% |
| Experimental Procedure Thermocouple thermometer,10 g of 1,10-dicyanodecane, 29.4 g of methanol, 0.36 g of a 28 wt% sodium methoxide-methanol solution (in methanol 3.0% by weight), and 0.27 g of a nickel catalyst developed in the same manner as in Example 1 was charged. After charging, hydrogen substitution was carried out, and thereafter the pressure was increased to 5 MPa. The temperature was elevated to an internal temperature of 130 ° C. over about 1 hour after boosting pressure.The reaction started at 130 ° C., and it was confirmed that almost no hydrogen consumption was consumed in about 3 hours. After further reacting for 1 hour, cooling down to room temperature, depressurizing, filtering the reaction solution to remove the catalyst, and washing the filtrate with 100 g of methanol. Methanol concentration was performed from the resultant methanol reaction solution of 1,12-diaminododecane. As a result, the concentration of crude 1,12-diaminododecane obtained after concentration was 10.34 g. The GC area% of 1,12-diaminododecane was 96.8 area%, and the reaction yield of 1,12-diaminododecane calculated from the quantitative value was 91.7%. |
Safety and Hazards
No data available
Other Data
| Transportation | Store in room temperature for long time; Away from light. |
| HS Code | |
| Storage | Store in room temperature for long time; Away from light. |
| Shelf Life | Half of a year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 200.368 |
| logP | 3.504 |
| HBA | 2 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 52.04 |
| Rotatable Bond (RotB) | 11 |
| Matching Veber Rules | 1 |
| Quantitative Results | ||
| 1 of 98 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | β2- adrenergic receptor agonists | |
| 2 of 98 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Propargyl amino compounds | |
| 3 of 98 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Bis[urea-urethane] compounds | |
| 4 of 98 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | ||
| 5 of 98 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | PYRROLE STUDIES PART 41. REACTIVITY OF 3,4-DIFORMYL-2,5-DIMETHYLPYRROLE WITH DIAMINOALKANES. AN UNUSUAL FORMATION OF 2-AZAFULVENES | |
| 6 of 98 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Synthesis and complexing properties of N,N,N’,N’-tetrakis-(8-hydroxy-5-quinolylmethyl)-α,ω-DIAMINOALKANES | |
| 7 of 98 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | SELF-ASSEMBLY OF STREPTAVIDIN/BISBIOTIN MONOLAYERS AND MULTILAYERS |
| Use Pattern |
| 1,12-Dodecanediamine CAS#: 2783-17-7 is one of the monomers for the production of nylon 1212. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |
