1,3-Bis(2,6-diisopropylphenyl)imidazolium chloride CAS#:250285-32-6; ChemWhat Code: 1411342
Identification
| Product Name | 1,3-Bis(2,6-diisopropylphenyl)imidazolium chloride |
| IUPAC Name | 1,3-bis[2,6-di(propan-2-yl)phenyl]imidazol-1-ium;chloride amine |
| Molecular Structure | 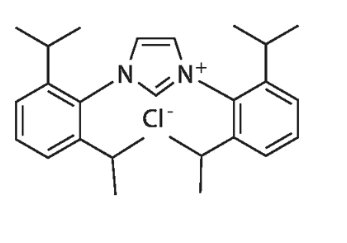 |
| CAS Registry Number | 250285-32-6 |
| EINECS Number | 627-434-9 |
| MDL Number | MFCD02684545 |
| Beilstein Registry Number | |
| Synonyms | 1,3-Bis(2,6-diisopropylphenyl)imidazolium chloride 250285-32-6 1,3-bis(2,6-diisopropylphenyl)-1h-imidazol-3-ium chloride 1,3-Bis(2,6-di-i-propylphenyl)imidazolium chloride 2,5-Bis(2,6-diisopropylphenyl)imidazolium chloride MFCD02684545 1,3-Bis[2,6-bis(1-methylethyl)phenyl]-1H-imidazolium chloride 1,3-bis[2,6-di(propan-2-yl)phenyl]imidazol-1-ium;chloride 1,3-bis[2,6-bis(propan-2-yl)phenyl]-3H-1lambda5-imidazol-1-ylium chloride 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride 1,3-Bis(2,6-di-i-propylphenyl)imidazoliumchloride SCHEMBL360316 YSZC1308 1,3-Bis(2,6-diisopropylphenyl)imidazolium chloride, 97% DTXSID40370572 1,3-Bis-(2,6-diisopropyl-phenyl)-3H-imidazol-1-ium chloride AMY14361 AKOS015909582 |
| Molecular Formula | C27H37ClN2 |
| Molecular Weight | 425 |
| InChI | InChI=1S/C27H37N2.ClH/c1-18(2)22-11-9-12-23(19(3)4)26(22)28-15-16-29(17-28)27-24(20(5)6)13-10-14-25(27)21(7)8;/h9-21H,1-8H3;1H/q+1;/p-1 |
| InChI Key | AVJBQMXODCVJCJ-UHFFFAOYSA-M |
| Canonical SMILES | CC(C)C1=C(C(=CC=C1)C(C)C)N2C=C[N+](=C2)C3=C(C=CC=C3C(C)C)C(C)C.[Cl-] |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2020/48925 | METHOD OF PREPARING METAL COMPLEXES OF FORMULA Z-M, IN PARTICULAR CARBENE-METAL COMPLEXES | 2020 |
| CN111100147 | Synthesis method of copper-nitrogen heterocyclic carbene complex catalyst | 2020 |
| WO2018/220003 | HYDROGENATION OF SUBSTITUTED FURANS CATALYZED BY NHC-CONTAINING LIGANDS | 2018 |
| CN107459451 | Preparation method of methyl-3-hydroxypropanoate | 2017 |
| CN107459453 | Preparation method of methyl-3-hydroxypropanoate | 2017 |
Physical Data
| Appearance | Off-white or white solid |
| Melting Point, °C | Solvent (Melting Point) |
| 250 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | 25 | |
| Chemical shifts, Spectrum | 13C | dimethylsulfoxide-d6 | 25 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| ATR (attenuated total reflectance), Bands | ||
| Bands | neat (no solvent) |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | acetonitrile |
Route of Synthesis (ROS)
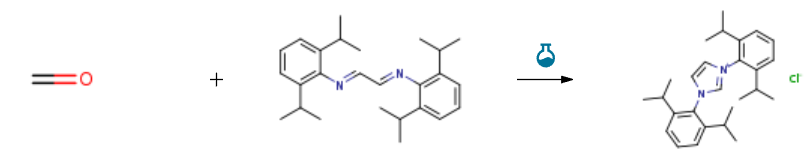
Route of Synthesis (ROS) of 1,3-Bis(2,6-diisopropylphenyl)imidazolium chloride CAS 250285-32-6
| Conditions | Yield |
| With chloro-trimethyl-silane In ethyl acetate at 70℃; for 2h; | 90% |
| With chloro-trimethyl-silane In ethyl acetate at 70℃; for 2h; | 89% |
| Experimental Procedure 4.3. Preparation of the catalyst i. A mixture of 2,6-diisopropylaniline (33.9 mmol) and HOAc (1.18 mmol) and 15 mL MeOH was stirred in at 50 °C, then slowly dropwise added 15 mL of a MeOH solution of glyoxal (40% aqueous solution, 16.7 mmol) in 15 minutes. After the addition, the mixture continuing to stir at 50 °C for 30 minutes and then at room temperature for 10 hours. The reaction mixture was filtered dried to obtain 5.6 g of yellow compound a[57] (87% yield). ii. a (8.5 mmol) and paraformaldehyde (8.5 mmol) were added into 30 mL EtOAc and stirred vigorously to dissolve at 70 °C. Then, 20 mL of TMSCl (0.85 mmol) in EtOAc was slowly added dropwise to the reaction flask within 20 minutes. After reacting for 2 h, the reaction mixture is cooled to 10 °C and filtered. The filter cake was washed with EtOAc and dried to obtain 3.2 g of compound b[57] as a white solid (89% yield). |
Safety and Hazards
| Pictogram(s) |     |
| Signal | Danger |
| GHS Hazard Statements | H300 (65.93%): Fatal if swallowed [Danger Acute toxicity, oral] H315 (34.07%): Causes skin irritation [Warning Skin corrosion/irritation] H317 (65.93%): May cause an allergic skin reaction [Warning Sensitization, Skin] H318 (65.93%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H319 (34.07%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (62.22%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] H400 (65.93%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410 (65.93%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P261, P264, P264+P265, P270, P271, P272, P273, P280, P301+P316, P302+P352, P304+P340, P305+P351+P338, P305+P354+P338, P317, P319, P321, P330, P332+P317, P333+P313, P337+P317, P362+P364, P391, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Store at room temperature for long time, Keep container tightly closed in a dry and well-ventilated place. |
| HS Code | |
| Storage | Store at room temperature for long time, Keep container tightly closed in a dry and well-ventilated place. |
| Shelf Life | 2 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 425.057 |
| logP | 9.81 |
| HBA | 1 |
| HBD | 0 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 8.81 |
| Rotatable Bond (RotB) | 6 |
| Matching Veber Rules | 2 |
| Bioactivity |
| Quantitative Results |
| Quantitative Results | ||
| 1 of 64 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Use of a catalyst system comprising nickel, palladium, or platinum and imidazoline-2-ylidene or imidazolidine-2-ylidene in kumada coupling reactions | |
| 2 of 64 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Zinc N-heterocyclic carbene complexes and their polymerization of d,l-lactide | |
| 3 of 64 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Reductive cyclization of diynes and enynes catalyzed by allyl platinum N-heterocyclic carbene complexes | |
| 4 of 64 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Concomitant formation of n-heterocyclic carbene-copper complexes within a supramolecular network in the self-assembly of imidazolium dicarboxylate with metal ions | |
| 5 of 64 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | NUCLEOPHILIC HETEROCYCLIC CARBENE DERIVATIVES OF PD(ACAC)2 FOR CROSS-COUPLING REACTIONS |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| Use Pattern |
| 1,3-Bis(2,6-diisopropylphenyl)imidazolium chloride CAS#:250285-32-6 is used in organophosphate ligands. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |
