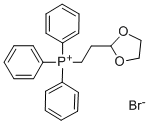
2-(1,3-Dioxolan-2-yl)ethyltriphenylphosphonium bromide CAS#: 86608-70-0; ChemWhat Code: 69059
Identification
Physical Data
| Appearance | White to Light yellow to Light orange solid |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | tetradeuteriomethanol | 400 |
| Spectrum | 1H | tetradeuteriomethanol | 400 |
| Spectrum | 13C | tetradeuteriomethanol | 100 |
| Chemical shifts | 13C | tetradeuteriomethanol | 100 |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | |
| Spin-spin coupling constants | dimethylsulfoxide-d6 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | KBr |
Route of Synthesis (ROS)
| Conditions | Yield |
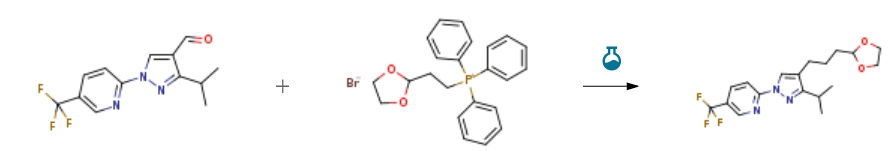
| Stage #1: 3-(1-methylethyl)-1-[5-(trifluoromethyl)pyridin-2-yl]-1H-pyrazole-4-carbaldehyde; 2-(1,3-dioxolan-2-yl)ethyl(triphenyl)phosphonium bromide With sodium hydride In DMF (N,N-dimethyl-formamide) at 0 – 70℃; Stage #2: With hydrogen; 5%-palladium/activated carbon In ethanol at 20℃; for 3.5h; Experimental Procedure Reference Example 52 Reference Example 52 To a mixture of2- (1, 3-dioxolan-2- yl) ethyltetraphenylphosphonium bromide (18.95 g) and N, N- dimethylformamide (178 ml) was added sodium hydride (60%, in oil, 1.71 g) at0 C and the mixture was stirred at room temperature for 30 minutes. Then,3-isopropyl-l- [5-(trifluoromethyl)-2-pyridinyl]-lH-pyrazole-4-carbaldehyde (10.09 g) was added and the mixture was stirred at room temperature overnight, and at70 C for 4 hours. The reaction mixture was poured into dilute hydrochloric acid, and extracted with ethyl acetate. The ethyl acetate layer was washed with saturated aqueous sodium chloride solution, dried(MgS04) and concentrated. The residue was subjected to silica gel column chromatography, and a colorless oil was obtained from a fraction eluted with ethyl acetate-hexane (1: 15, volume ratio). A mixture of the obtained oily substance,5% palladium-carbon (1.28 g) and ethanol (174 ml) was stirred at room temperature for 3.5 hours under a hydrogen atmosphere. Palladium-carbon was removed by filtration and the filtrate was concentrated to give2- {4- [3- (1, 3-dioxolan-2-yl)propyl-3-isopropyl-lH-pyrazol-1-yl}-5-(trifluoromethyl) pyridine (12.84 g, yield98%) as a colorless oil. 1H-NMR (CDC13) g : 1.32 (6H, d, J = 7.0 Hz), 1.72-1. 82 (4H, m), 2.46-2. 58 (2H, m), 2.92-3. 10 (1H, m), 3.82-4. 00 (4H, m), 4.88-4. 96 (1H, m), 7.88-7. 98 (1H, m), 8.02 (1H, d, J = 8.4 Hz), 8.27 (1H, s), 8.56-8. 61 (1H, m). | 98% |
Safety and Hazards
| Pictogram(s) |    |
| Signal | Danger |
| GHS Hazard Statements | H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] H318 (100%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H373 (100%): May causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] H412 (100%): Harmful to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P260, P264, P264+P265, P270, P273, P280, P301+P317, P305+P354+P338, P317, P319, P330, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under room temperature away from light |
| Storage | Under room temperature away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 443.32 |
| logP | 7.198 |
| HBA | 2 |
| HBD | 0 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 18.46 |
| Rotatable Bond (RotB) | 6 |
| Matching Veber Rules | 2 |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |