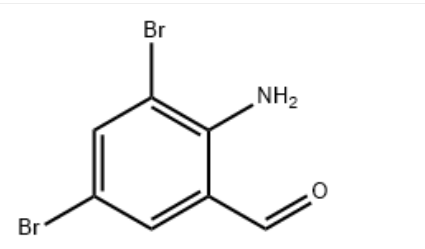
2-Amino-3,5-dibromobenzaldehyde CAS#: 50910-55-9; ChemWhat Code: 1411689
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN115466212 | 2-trifluoromethyl quinoline compound as well as synthesis method and application thereof | 2022 |
| CN110684031 | Asymmetric synthesis method of trans-tetrahydropyrine and tetrahydroquinoline derived chiral compound | 2020 |
| CN109096196 | Preparation method and application of 2-amino-3,5-bibromobenzyl intermediate compound | 2018 |
Physical Data
| Appearance | Yellow Crystalline Powder |
| Melting Point, °C | Solvent (Melting Point) |
| 136.2 – 138.6 | |
| 137 – 139 | ethanol |
| 135 – 136 | ethanol |
| 137 – 137.5 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | 400 | |
| Chemical shifts, Spectrum | 13C | dimethylsulfoxide-d6 | 125 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 500 | |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 125 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 300 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | KBr |
| Bands | CHCl3 |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Absorption maxima | methanol | 234, 261.5 |
Route of Synthesis (ROS)
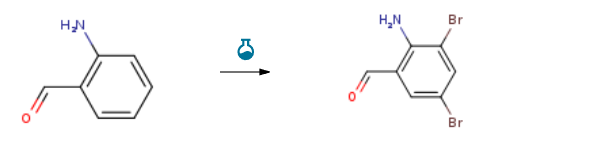
Route of Synthesis (ROS) of 2-Amino-3,5-dibromobenzaldehyde CAS 50910-55-9
| Conditions | Yield |
| With bromine; potassium bromide In water | 91.1% |
| With bromine; potassium bromide In ethanol; water at 20℃; for 1h; Temperature; | 91.9% |
| With bromine; potassium bromide In ethanol; water for 1h; Ambient temperature; | 80% |
| Experimental Procedure Stir and mix ethanol and o-aminobenzaldehyde at a molar ratio of 5:1;2. Add a solution of bromine, potassium bromide and water to the reaction solution with stirring, wherein the molar ratio of o-aminobenzaldehyde, bromine and potassium bromide is 1:1.9:9, and the dripping time is 40 minutes;3. Stir the reaction at 20°C for 1 hour and follow the progress of the reaction with high performance liquid chromatography (HPLC);4. Continue adding excess saturated sodium bicarbonate solution to the reaction mixture and stir until solid precipitates and obtain 2-amino-3,5-dibromobenzaldehyde by filtration. The yield of 2-amino-3,5-dibromobenzaldehyde was 91.9%. |
Safety and Hazards
No data available
Other Data
| Transportation | Keep material sealed and store in cool, dry place, away from sunlight and moisture. |
| HS Code | |
| Storage | Keep material sealed and store in cool, dry place, away from sunlight and moisture. |
| Shelf Life | 2 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 278.931 |
| logP | 2.926 |
| HBA | 2 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 43.09 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| Use Pattern |
| 2-Amino-3,5-dibromobenzaldehyde CAS#: 50910-55-9 can serve as an intermediate in organic synthesis. It can participate in various chemical reactions such as amination, electrophilic substitution, condensation reactions, etc., to construct the framework of complex organic molecules or synthesize target compounds. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |