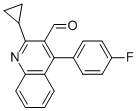
2-Cyclopropyl-4-(4-fluorophenyl)quinoline-3-carboxaldehyde CAS#: 121660-37-5; ChemWhat Code: 340124
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN107226814 | Preparation method for intermediate of Baricitinib | 2017 |
| US2012/225812 | SUBSTITUTED FUSED PYRIMIDINE COMPOUNDS, ITS PREPARATION AND USES THEREOF | 2012 |
| WO2009/106577 | IMIDAZO [1,2-B] PYRIDAZINE DERIVATIVES FOR THE TREATMENT OF C-MET TYROSINE KINASE MEDIATED DISEASE | 2009 |
| WO2006/40964 | METHOD OF PRODUCING SILYLALKOXYMETHYL HALIDE | 2006 |
Physical Data
| Appearance | Off-white or slightly yellow crystalline powder |
| Melting Point, °C |
| 145 – 147 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 57 – 59 | 8 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.05 | 25 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Spectrum | 300 | ||
| Chemical shifts, Spectrum | 13C | ||
| Chemical shifts | 13C | ||
| Chemical shifts | 1H | acetone-d6 | 300 |
| 1H | acetone-d6 | 300 | |
| Chemical shifts | 1H | CDCl3 | |
| Chemical shifts | 13C | CDCl3 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | KBr |
Route of Synthesis (ROS)
| Conditions | Yield |
| Stage #1: Indole-3-carboxaldehyde With sodium hydride In N,N-dimethyl-formamide; mineral oil at 20℃; for 1h; Inert atmosphere; Stage #2: (2-trimethylethylsilylethoxy)methyl chloride In N,N-dimethyl-formamide for 2h; | 98% |
| Stage #1: Indole-3-carboxaldehyde With sodium hydride In N,N-dimethyl-formamide at 0 – 20℃; Inert atmosphere; Stage #2: (2-trimethylethylsilylethoxy)methyl chloride at 0 – 20℃; Experimental Procedure 2.4. Typical procedure for the preparation of 1-((2-(trimethylsilyl)ethoxy)methyl)-1H-indole-3-carbaldehydes: synthesis of the 1-((2-(trimethylsilyl)ethoxy)methyl)-1H-indole-3-carbaldehyde To a stirred suspension of NaH (331 mg, 8.27 mmol, 1.2 equiv.) in DMF anhydrous (5 mL) was added dropwise a solution of 1H-indole-3-carbaldehyde (1.0 g, 6.89 mmol, 1.0 equiv.) dissolved in anhydrous DMF (10.0 mL) at 0°C under argon. The mixture was stirred for 1 hour, warmed to room temperature and stirred until the salt was formed. Then, reaction mixture was cooled to 0°C before adding dropwise SEMCl (1.40 mL, 7.58mmol, 1.1 equiv.), warmed to room temperature and stirred for 10 minutes. After the consumption of the substrate (TLC, n-hexane-EtOAc, 80:20), the reaction was diluted with Et2O, washed with a solution of KHSO4 (10% w/w), a saturated solution of NaHCO3, and brine. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by chromatography on SiO2 (25-40 μm), eluting with 85/15 (v/v) n-hexane/AcOEt mixture (Rf = 0.22) to obtain 1.85 g (97% yield) of 1-((2-(trimethylsilyl)ethoxy)methyl)-1H-indole-3-carbaldehyde. | 97% |
| With sodium hydride Substitution; | 85% |
Safety and Hazards
| Precautionary Statement Codes | Not Classified |
Other Data
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 166.723 |
| logP | 2.264 |
| HBA | 1 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 9.23 |
| Rotatable Bond (RotB) | 4 |
| Matching Veber Rules | 2 |
| Use Pattern |
| As an intermediate, 2-Cyclopropyl-4-(4-fluorophenyl)quinoline-3-carbaldehyde helps to enhance the synthesis efficiency of Pitavastatin calcium, making the production process more efficient and controllable. The quality and purity of the intermediate directly affect the final drug’s quality. High-quality intermediates contribute to producing Pitavastatin calcium with higher purity and fewer side effects. Choosing the appropriate intermediate in the drug synthesis process can minimize unnecessary by-products, thereby reducing the final drug’s side effects. The structure of 2-Cyclopropyl-4-(4-fluorophenyl)quinoline-3-carbaldehyde contributes to the pharmacological activity of Pitavastatin calcium, making it more effective in lowering cholesterol and preventing cardiovascular diseases. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |