2-Hydroxyethyl acrylate CAS#: 818-61-1; ChemWhat Code: 57446
Identification
| Product Name | 2-Hydroxyethyl acrylate |
| IUPAC Name | 2-hydroxyethyl prop-2-enoate |
| Molecular Structure | |
| CAS Registry Number | 818-61-1 |
| EINECS Number | 212-454-9 |
| MDL Number | MFCD00002865 |
| Beilstein Registry Number | 969853 |
| Synonyms | 2-hydroxyethyl acrylate, hydroxyethyl acrylate |
| Molecular Formula | C5H8O3 |
| Molecular Weight | 116.12 |
| InChI | InChI=1S/C5H8O3/c1-2-5(7)8-4-3-6/h2,6H,1,3-4H2 |
| InChI Key | OMIGHNLMNHATMP-UHFFFAOYSA-N |
| Canonical SMILES | C=CC(=O)OCCO |
Physical Data
| Appearance | Colorless transparent flowable liquid |
| Boiling Point | 100 °C |
| Refractive index | n20/D 1.45(lit.) |
| Water Solubility | soluble |
| Sensitivity | Light Sensitive |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 83 – 85 | 10 |
| 66 | 1.1 |
| 45 | 5 |
| 65 – 67 | 3 |
| 77 – 78 | 4 |
| 75 – 77 | 2 |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C |
| 1.4452 | 589 | 23 |
| 1.451 | 589 | 20 |
| 1.442 | 589 | 20 |
| 1.448 | 589 | 25 |
| 1.446 | 589 | 23 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.1076 | 4 | 20 |
| 1.077 | 20 | 20 |
| 1.1091 | 4 | 20 |
| 1.011 | 4 | 23 |
| Bulk Viscosity, P | Temperature (Bulk Viscosity), °C |
| 0.0605 | 25 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | water-d2 | ||
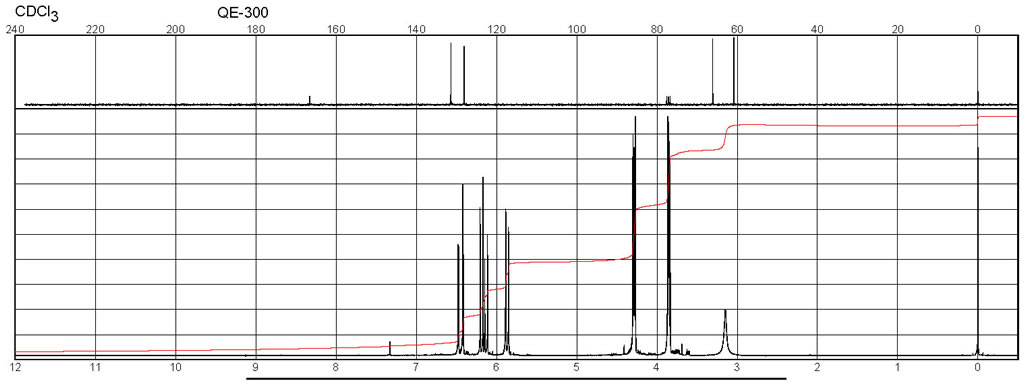
| Spectrum | 1H | water-d2 | 400 | |
| Chemical shifts | 1H | chloroform-d1 | 300 | |
| Chemical shifts | 13C | chloroform-d1 | 125 | |
| Chemical shifts | 1H | 300 | ||
| Chemical shifts | 13C | CD2Cl2 | 25 | 75 |
| Spectrum | 1H | CDCl3 | 24.85 | |
| Chemical shifts | 1H | CCl4 | 20-26 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C | Comment (IR Spectroscopy) |
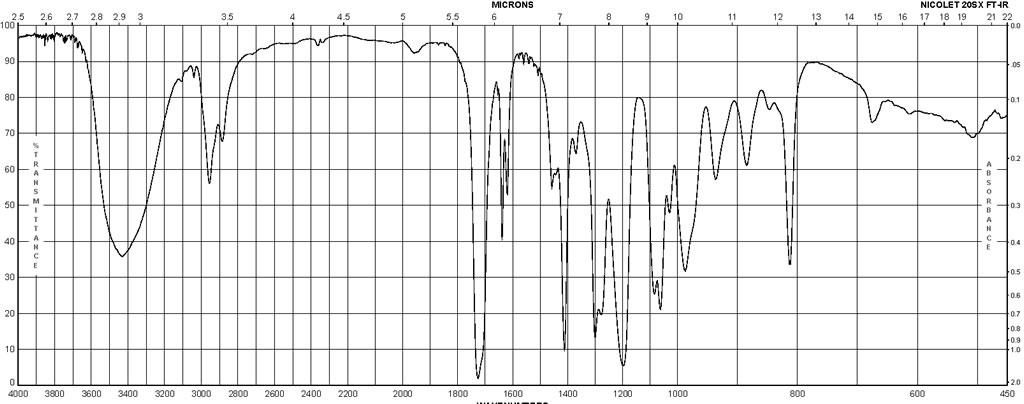
| ATR (attenuated total reflectance), Bands, Spectrum | |||
| Bands | film | ||
| Intensity of IR bands, Bands, Spectrum | potassium bromide | ||
| FT-IR-Difference Spectroscopy, Bands, Spectrum | potassium bromide | ||
| Spectrum | 25 | temperature dependence | |
| Spectrum | 120 | 3440 – 146 cm**(-1) | |
| Spectrum | tetrahydrofuran | 3800 – 3100 cm**(-1) | |
| Bands | tetrahydrofuran | 3432 cm**(-1) | |
| Bands | CCl4 | 3623 – 3460 cm**(-1) |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| 206, 247 |
Route of Synthesis (ROS)
| Conditions | Yield |
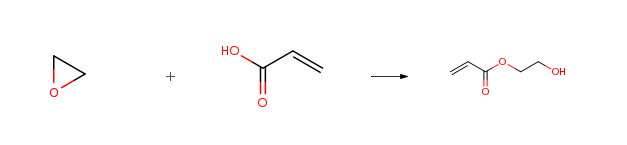
| With magnetic zeolite at 63℃; for 2h; Temperature; Experimental Procedure 100 g of acrylic acid was added to the reaction kettle, Then add 4g magnetic zeolite molecular sieve, stirred evenly, Add 59 grams of ethylene oxide, turn off the reactor, heated to 63 °C, After 2 hours of reaction, distillation was carried out under reduced pressure to obtain 151.8 g of hydroxyethyl acrylate (Yield 97.5percent, purity 99.0percent). | 97.5% |
| With chromium(lll) acetate; 4-methoxy-phenol; hydroquinone In water at 80℃; under 5625.56 Torr; for 1.5h; Temperature; Inert atmosphere; Experimental Procedure Under vacuum, vacuum 2000kg acrylic acid, 2.0percent by mass of chromium acetate, 13 × 10-4 hydroquinone and p-hydroxyanisole, after completion of the vacuum to ensure 0.75Mpa, measuring tank nitrogen After the replacement, ethylene oxide was added to ensure that the volume ratio of oxygen to nitrogen was 0.25percent, the ethylene oxide was added dropwise while the steam was heated to 80 ° C, the pressure was controlled by controlling the ethylene oxide dropping rate to be less than or equal to 60KPa, the ethylene oxide The molar ratio of acrylic acid to acrylic acid is 1.02: 1, the reaction temperature is controlled at 90 ° C. and the reaction time is 1.5 hours by cooling with cooling water; cooling water is shut down after the completion of the dropwise addition and naturally warmed; when the temperature drops, the mass percent of the acrylic acid sampled and detected is less than 0.5percent The reaction is completed; The film is evaporated under vacuum condition to obtain the primary distillation product, and the catalyst and the heavy component enter the residual liquid tank from the bottom of the thin film evaporator; the primary distillation product obtained from the weight removal enters the rectification column for rectification and purification operation, , In the process of phase change through the way of the spray inhibitor polymerization inhibitor was added to a distillation of hydroxyethyl acrylate, and added to block the air, while controlling the distillation tower overhead vacuum of 0.5KPa, the operating temperature is 85 , the reflux ratio of 1.1, through the top of the distillation tower discharge to obtain high-purity hydroxyethyl acrylate, tower reactor containing hydroxyethyl acrylate and some of the heavies distillation residue, and then after secondary distillation purification separation Hydroxyethyl acrylate and distillation residue, the rectification residue is sent to the raffinate treatment device, the raffinate is processed by a thin film evaporator, and the hydroxyethyl acrylate in the raffinate is recovered.The gas chromatogram of the product obtained in this example is shown in Figure 1, and the area normalization method is shown in Table 1. The retention time of 2.238min is the peak of hydroxyethyl acrylate with the content of 97.91percent; the retention time The di-ester peak at 2.963 min, content of 0.41percent. | 97.91% |
| With 10H-phenothiazine; chromium acetate at 80 – 90℃; under 750.075 Torr; for 3.7 – 5.2h; | 81% |
Safety and Hazards
| Pictogram(s) |     |
| Signal | Danger |
| GHS Hazard Statements | H311: Toxic in contact with skin [Danger Acute toxicity, dermal] H314: Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] H317: May cause an allergic skin reaction [Warning Sensitization, Skin] H400: Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] |
| Precautionary Statement Codes | P260, P261, P264, P272, P273, P280, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P322, P333+P313, P361, P363, P391, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | No data vailable |
| Market Price | No data vailable |
| Use Pattern |
| Pharmaceuticals |
| 2-Hydroxyethyl acrylate CAS#: 818-61-1 is a monomer for polymer shell of protein nanocapsule |
| Hydrophilic vinylic monomer for production polymer of soft contact lens |
| 2-Hydroxyethyl acrylate CAS#: 818-61-1 can be dental gel etching composition |
| bonding adhesive agent |
| polymerizable ethylenically unsaturated monomer |
| preparation of polymerizable mono(meth)acrylate resin useful as a component of curable dental restorative composition |
| water miscible monomer |
| ultraviolet light curable formulation for composite repair |
| functional monomer for preparing a adhesive coating of the composition for transdermal delivery of estradiol |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |