2-Hydroxyethyl methacrylate CAS#: 868-77-9; ChemWhat Code: 55281
Identification
| Product Name | 2-Hydroxyethyl methacrylate |
| IUPAC Name | 2-hydroxyethyl 2-methylprop-2-enoate |
| Molecular Structure | 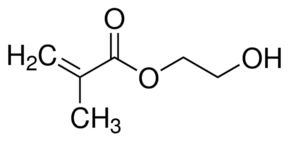 |
| CAS Registry Number | 868-77-9 |
| EINECS Number | 212-782-2 |
| MDL Number | MFCD00002863 |
| Beilstein Registry Number | 1071583 |
| Synonyms | 2-methyl-2-propenoic acid 2-hydroxyethyl ester, 2-Hydroxyethyl methacrylate, ethylene glycol monomethacrylate |
| Molecular Formula | C2H5N3O2 |
| Molecular Weight | 103.081 |
| InChI | InChI=1S/C2H5N3O2/c3-1(6)5-2(4)7/h(H5,3,4,5,6,7) |
| InChI Key | WOBHKFSMXKNTIM-UHFFFAOYSA-N |
| Canonical SMILES | CC(=C)C(=O)OCCO |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN109704989 | A phthalic nitrile acrylic ester monomer synthesis method (by machine translation) | 2019 |
| KR2019/47223 | PHOTO CURABLE ISOSORBIDE DERIVATIVE COMPOUND AND METHOD FOR PREPARING THE SAME | 2019 |
| JP2018/62507 | Compound, resin, and a method for manufacturing a resist pattern a resist composition (by machine translation) | 2018 |
| CN105153223 | For 3 D SLA printing of the phosphorus-containing acrylate prepolymer and its preparation method (by machine translation) | 2018 |
| US2017/145216 | RHODAMINE-BASED COLORING COMPOSITION | 2017 |
| JP6093949 | The benzotriazole derivative compound and polymer (by machine translation) | 2017 |
| WO2016/46292 | LIQUID THIOETHER CARBOXYLIC ACID ESTERS | 2016 |
| US8791290 | Acetal compound, polymer, resist composition, and patterning process | 2014 |
| US2004/171867 | Reactive monomer composition modified by a small-amount of lactones, an acrylic polyol resin, a curable resin composition, and a coating composition | 2004 |
Physical Data
| Appearance | Colorless transparent flowable liquid |
| Water Solubility | soluble |
| Refractive index | n20/D 1.453(lit.) |
| Sensitivity | Air Sensitive |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 86 – 88 | 5.0255 |
| 83 – 85 | 20 |
| 87 | 5 |
| 80 | 3 |
| 79 | 4 |
| 226 | |
| 115 | 20 |
| 79 | 4.00008 |
| 55 | 3 |
| 75 – 76 | 2 |
| 103 | 13 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.0486 – 1.0671 | 24.99 – 44.99 |
| 1.0486 – 1.0671 | 25-45 |
| 1.0486 – 1.0747 | 15-45 |
| 1.077 | 20 |
| 1.0715 | 20 |
| 1.0678 | 20 |
| 1.079 | 20 |
| 1.0712 | 20 |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C | Comment (Refractive Index) |
| 1.45 | 589 | 20 | |
| diagram. Object(s) of Study: temperature dependence | |||
| 1.4481 | 589 | 20 | |
| 1.4515 | 589 | 20 | |
| 1.4451 | 589 | 20 | |
| 1.4527 | 589 | 20 |
| Description (Association (MCS)) | Original string |
| GC (Gas chromatography) | HEMA 14.86 minutes |
| GC (Gas chromatography) |
| Description (Compressibility) | Comment (Compressibility) |
| Adiabatic compressibility | diagram. Object(s) of Study: temperature dependence |
| Dynamic Viscosity, P | Temperature (Dynamic Viscosity), °C | Comment (Dynamic Viscosity) |
| 0.03181 – 0.05784 | 24.99 – 44.99 | |
| 0.03181 – 0.05784 | 25-45 | |
| 0.03181 – 0.08234 | 15-45 | |
| diagram. Object(s) of Study: temperature dependence | ||
| 0.05784 – 31.81 | 25-45 |
| Description (Transport Phenomena (MCS)) | Temperature (Transport Phenomena (MCS)), °C | Partner (Transport Phenomena (MCS)) |
| Dynamic viscosity | 25 – 45 | cyclohexanone |
| Dynamic viscosity | 25 – 45 | propylene glycol methyl ether acetate |
| Dynamic viscosity | 25 – 45 | ethyl 3-ethoxypropionate |
| Dynamic viscosity | 25 | H2O |
| Dynamic viscosity | 80 | H2O |
| Description (Liquid/Solid Systems (MCS)) | Partner (Liquid/Solid Systems (MCS)) |
| Glass transition temperature(s) | D-glucose, (2R,3R,4S,5S,6R)-2-Ethoxy-6-hydroxymethyl-tetrahydro-pyran-3,4,5-triol, propyl β-D-glucopyranoside, propan-1-ol |
| Glass transition temperature(s) | D-Galactose, 1-O-ethyl-β-D-galactopyranoside, n-propyl-β-D-galactopyranoside |
| Description (Liquid/Vapour Systems (MCS)) | Pressure (Liquid/Vapour Systems (MCS)), Torr | Partner (Liquid/Vapour Systems (MCS)) |
| Boiling points of mixtures | 20627.1 – 157816 | carbon dioxide |
| Critical temperature | ||
| Critical pressure |
| Description (Mechanical & Physical Properties (MCS)) | Temperature (Mechanical & Physical Properties (MCS)), °C | Comment (Mechanical & Physical Properties (MCS)) | Partner (Mechanical & Physical Properties (MCS)) |
| Volume change on mixing | 35 | diagram | cyclohexanone |
| Volume change on mixing | 15-45 | butan-1-ol | |
| Volume change on mixing | 35 | diagram | ethyl 3-ethoxypropionate |
| Description (Sound Properties) | Comment (Sound Properties) |
| Hypersonic velocity | diagram. Object(s) of Study: temperature dependence |
Spectra
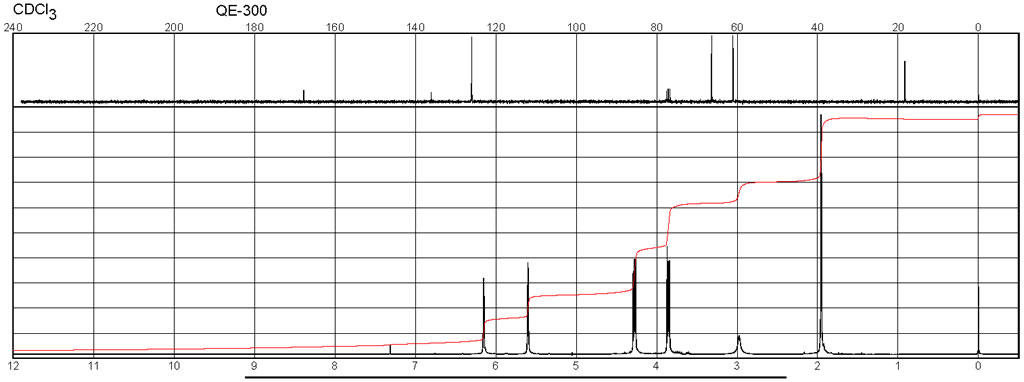
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts,Spectrum | 1H | d(4)-methanol | 25 | 500 |
| Chemical shifts,Spectrum | 1H | dimethylsulfoxide-d6 | ||
| Chemical shifts ,Spectrum | 1H | chloroform-d1 | ||
| Chemical shifts ,Spectrum | 13C | chloroform-d1 | ||
| Chemical shifts | 13C | chloroform-d1 | ||
| Chemical shifts | 1H | chloroform-d1 | 200 | |
| Chemical shifts | 1H | CCl4 | 20-26 |
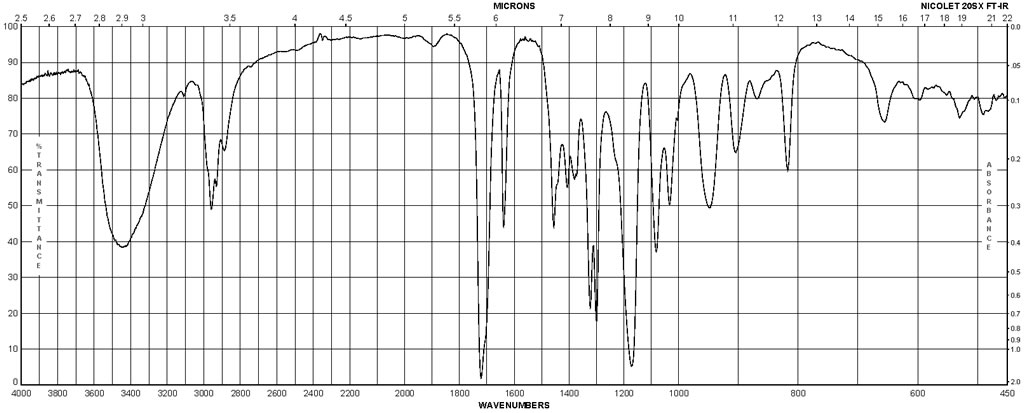
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C | Comment (IR Spectroscopy) |
| ATR (attenuated total reflectance), Bands, Spectrum | |||
| Bands, Spectrum | potassium bromide | ||
| Intensity of IR bands, Bands, Spectrum | 20 | ||
| Bands | potassium bromide | ||
| Bands, Spectrum | film | ||
| Spectrum | CCl4 | 25 | 3200 – 1800 cm**(-1) |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| 207, 259 |
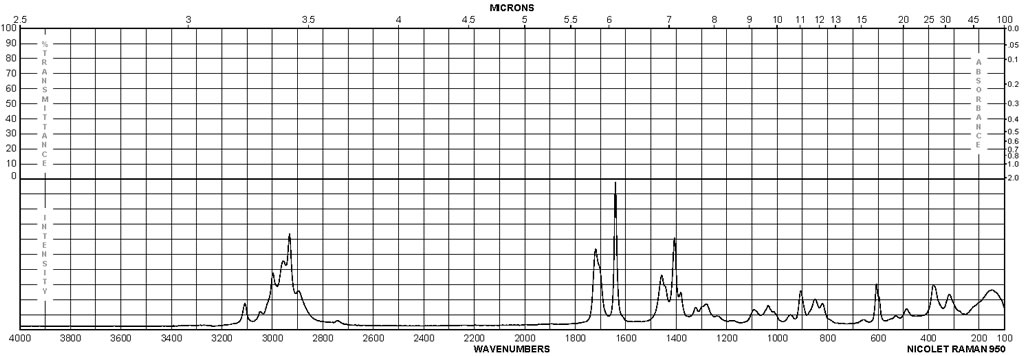
| Description (Raman Spectroscopy) | Solvent (Raman Spectroscopy) | Comment (Raman Spectroscopy) |
| Bands, Spectrum |
Route of Synthesis (ROS)
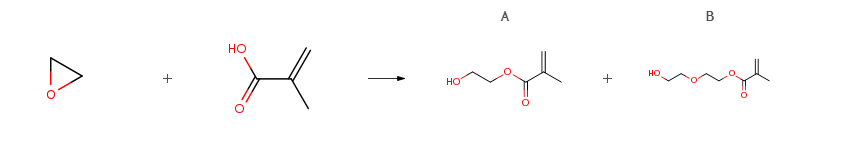
| Conditions | Yield |
| With triethanolamine; poly(ferric methacrylate); choline chloride In water at 30 – 66℃; for 5.53h; Experimental Procedure A mixed solution (solution C) of 2.79 g (0.019 mol) of choline chloride and 0.93 g (0.049 mol) of water was prepared at room temperature. Furthermore, a solution (solution D) of 2.98 g (0.020 mol) of triethanolamine and 0.053 g of a benzyl ester form of HO-TEMPO as a polymerization inhibitor dissolved in 61.0 g (0.709 mol) of methacrylic acid (MAA) was prepared at room temperature. Subsequently, the solution C and the solution D were introduced into a 1-L pressurized reactor made of SUS, and then 427.9 g of the iron(III) methacrylate solution obtained in Example 21 was introduced into the pressurized reactor made of SUS. While this mixed solution was stirred, 30 g (0.68 mol) of ethylene oxide (EO) was added dropwise thereto over 7 minutes at 30°C, and subsequently, 300 g (6.81 mol) of EO was added dropwise thereto over 115 minutes at 66°C. This reaction liquid was stirred for 3.5 hours at 66°C, and then was cooled to 50°C. The EO remaining in the reaction liquid was removed under reduced pressure (11.3 kPa) over 1.5 hours. As a result, a 2-hydroxyethyl methacrylate solution in which the amount of residual methacrylic acid in the liquid was 0.4percent by mass, the amount of ethylene glycol dimethacrylate produced as a side product was 1.0percent by mass, and the amount of diethylene glycol monomethacrylate was 5.4percent by mass, was obtained. At this time, the reaction yield of 2-hydroxyethyl methacrylate was 93.9percent (on the basis of the moles of the raw material methacrylic acid). | A 93.9% B n/a |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315: Causes skin irritation [Warning Skin corrosion/irritation] H317: May cause an allergic skin reaction [Warning Sensitization, Skin] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] |
| Precautionary Statement Codes | P261, P264, P272, P280, P302+P352, P305+P351+P338, P321, P332+P313, P333+P313, P337+P313, P362, P363, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | No data vailable |
| Market Price | No data vailable |
| Use Pattern |
| 2-Hydroxyethyl methacrylate CAS#: 868-77-9 can be used in cosmetics/dental/toilet |
| Adhesion promoter in dental restorative composition |
| Cement composition for bonding a dental prosthetic appliance to a tooth |
| Cross-linking polymerizable component of a two-part resin-reinforced cement |
| In combination with an amine activator component |
| kit for temporarily bonding a provisional dental prosthetic appliance to a tooth |
| A composition for forming a coating on a nail in combination with a curable resin, a photoinitiator, and a chemical filter capable of absorbing ultraviolet (UV) radiation and reducing exotherm |
| Curable monomer for fiber-reinforce composites used for dental applications |
| Curable monomer for fiber-reinforce composites used for nails and joints for hip, knee and shoulder |
| Monomer in a matrix controlled diffusion drug delivery systems for treating retinitis pigmentosa |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |