2-Iodoacetamide CAS#: 144-48-9; ChemWhat Code: 24510
Identification
| Product Name | 2-Iodoacetamide |
| IUPAC Name | 2-iodoacetamide |
| Molecular Structure | 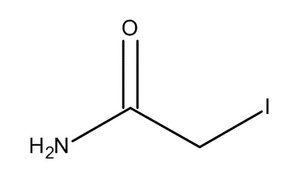 |
| CAS Registry Number | 144-48-9 |
| EINECS Number | 205-630-1 |
| MDL Number | MFCD00008028 |
| Beilstein Registry Number | No data available |
| Synonyms | iodacetamide, iodoacetamid |
| Molecular Formula | C2H4INO |
| Molecular Weight | 184.96 |
| InChI | InChI=1S/C2H4INO/c3-1-2(4)5/h1H2,(H2,4,5) |
| InChI Key | PGLTVOMIXTUURA-UHFFFAOYSA-N |
| Canonical SMILES | C(C(=N)O)I |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2013/65913 | COMPOUNDS | 2013 |
| WO2013/115661 | NEW QUATERNARY AMMONIUM SALTS, METHOD FOR PREPARATION AND APPLICATIONS THEREOF | 2013 |
| EP2077261 | METHOD FOR PRODUCING SUCCINIMIDE COMPOUND | 2009 |
| EP1612204 | HYDRAZONE DERIVATIVE | 2006 |
Physical Data
| Appearance | White Crystalline |
| Solubility | H2O: 0.5 M at 20 °C, clear, colorless |
| Flash Point | 88 ºC |
| Refractive index | 1.5560 (estimate) |
| Sensitivity | Light Sensitive |
| Melting Point, °C | Solvent (Melting Point) |
| 92 – 94 | benzene |
| 93 – 94 | H2O |
| 93 – 94 | CHCl3 |
| 95 | H2O |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Further physical properties of the complex | H2O | 25 | 1-[6-(dimethylamino)naphthalen-2-yl]prop-2-en-1-one |
| Further physical properties of the complex | bovine serum albumin | ||
| Further physical properties of the complex | H2O | -196.1 – -123.1 | DNA |
| Further physical properties of the complex | D2O, tetradeuteriomethanol | -196.1 – -123.1 | DNA |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| MAS (Magic-Angle Spinning), Chemical shifts, Spectrum | 13C | ||
| Chemical shifts | 13C | CDCl3 | 100.6 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | solid | 3520 – 425 cm**(-1) |
| Bands | gas | 3520 – 425 cm**(-1) |
| Bands | neat (no solvent) | 3520 – 425 cm**(-1) |
| IR |
| Description (Mass Spectrometry) |
| gas chromatography mass spectrometry (GCMS), electron impact (EI), tandem mass spectrometry, spectrum |
| electrospray ionisation (ESI), spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | H2O | 220 – 280 nm | ||
| Spectrum | 230 – 300 nm |
Route of Synthesis (ROS)
| Conditions | Yield |
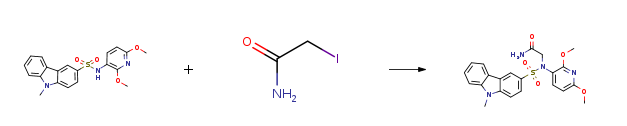
| With sodium hydride In N,N-dimethyl-formamide at 70℃; for 8h; Experimental Procedure 1 Example 1: N-(2,6-Dimethoxypyridin-3-yl)-N-(acetamido-2-yl)-9-methyl-3-carbazolesulfonamide (1) Preparation of N-(2,6-dimethoxypyridin-3-yl)-9-methyl-3-carbazolesulfonamide (IMB105) according toMitsumori, Susuμ Tsuri, Tatsuo; Honma, Tsunetoshi et al.The method of Journal of Medicinal Chemistry (2003), 46(12), 2436-2445 was synthesized.Dissolve N-(2,6-dimethoxypyridin-3-yl)-9-methyl-3-carbazolesulfonamide (IMB105) (0.30 g, 0.78 mmol) in 5 mL of dry DMF.Iodoacetamide (0.20 g, 1.1 mmol) and sodium hydride (40 mg, 60% in oil, 1.0 mmol) were added,The reaction was carried out at 70°C for 8 h. The reaction was complete by TLC.DMF was removed under reduced pressure, the residue was extracted with methylene chloride, and the water and saturated brine were washed sequentially and dried over anhydrous sodium sulfate.The mixture was filtered and purified by column chromatography on the filtrate (CDM/MeOH/concentrated aqueous ammonia = 40/1/0.1) to give 0.35 g of a solid (yield 98%). | 98% |
| Stage #1: N-(2,6-dimethoxypyridine-3-yl)-9-methyl-9H-3-carbazole sulfonamide With sodium hydride In N,N-dimethyl-formamide; mineral oil for 0.5h; Inert atmosphere; Cooling with ice; Stage #2: iodacetamide In N,N-dimethyl-formamide; mineral oil at 70℃; for 12h; Inert atmosphere; Experimental Procedure 4.1.6 2-((N-(2,6-dimethoxypyridin-3-yl)-9-methyl-9H-carbazole)-3-sulfonamido)acetamide (3q) IMB105 (310mg, 0.78mmol) was dissolved in dried DMF (5.0mL). The obtained solution was cooled on an ice-water bath. NaH (40mg, 1.0mmol, 60% in oil) was added to the solution, and the reaction mixture was stirred for 30min. Then 2-iodoacetamide (202mg, 1.1mmol) was added and the mixture was allowed to warm to 70°C and stirred for 12h. The solvent was removed in vacuo, and the obtained residue was extracted into ethyl acetate (100mL). The organic layer was washed consecutively with water (50mL), brine (50mL), dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified on silica gel using dichloromethane/methanol (20:1) to afford 350mg (98%) of 3q as a white solid. 1HNMR (CDCl3, 400MHz) δ ppm 3.68 (3H, s), 3.89 (3H, s), 3.92 (3H, s), 4.13 (2H, m), 6.25 (1H, d, J=8.4Hz), 7.15 (1H, d, J=8.4Hz), 7.34 (1H, t, J=8.0Hz), 7.36 (1H, br s), 7.49 (1H, d, J=8.8Hz), 7.53 (1H, d, J=8.0Hz), 7.57 (1H, td, J=8.4, 1.6Hz), 7.76(1H, dd, J=8.4, 1.6Hz), 8.10 (1H, d, J=8.0Hz), 8.42 (1H, d, J=2.0Hz); 13CNMR (DMSO-d6, 100MHz) δ ppm 29.3, 51.9, 52.9, 53.5, 101.0, 109.1, 109.8, 114.3, 119.9, 120.4, 121.1, 124.7, 126.8, 129.0, 141.4, 142.5, 144.5, 158.3, 161.6, 169.6; HRMS (ESI+): m/z calcd for C22H23N4O5S [M+H]+: 455.1377; found: 455.1384. | 98% |
Safety and Hazards
| Pictogram(s) |    |
| Signal | Danger |
| GHS Hazard Statements | H301 (98.88%): Toxic if swallowed [Danger Acute toxicity, oral] H317 (93.26%): May cause an allergic skin reaction [Warning Sensitization, Skin] H334 (88.76%): May cause allergy or asthma symptoms or breathing difficulties if inhaled [Danger Sensitization, respiratory] H413 (66.29%): May cause long lasting harmful effects to aquatic life [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P270, P272, P273, P280, P285, P301+P310, P302+P352, P304+P341, P321, P330, P333+P313, P342+P311, P363, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not Classified |
| Under the room temperature and away from light | |
| HS Code | 292419 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Use Pattern |
| 2-Iodoacetamide CAS#: 144-48-9 as Pharmaceuticals |
| 2-Iodoacetamide CAS#: 144-48-9 inhibiting virulence of A. baumannii bacteria in combination with an antibiotic |
| 2-Iodoacetamide CAS#: 144-48-9 inhibiting virulence of E. faecium bacteria in combination with an antibiotic |
| inhibiting virulence of Gram-negative bacteria in combination with an antibiotic |
| inhibiting virulence of Gram-positive bacteria in combination with an antibiotic |
| inhibiting virulence of K. pneumoniae bacteria in combination with an antibiotic |
| inhibiting virulence of P. aerugino bacteria in combination with an antibiotic |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watsonnoke Scientific Ltd | https://www.watsonnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |