2-Methyl-4′-(methylthio)-2-morpholinopropiophenone CAS#: 71868-10-5; ChemWhat Code: 50594
Identification
| Product Name | 2-Methyl-4′-(methylthio)-2-morpholinopropiophenone |
| IUPAC Name | 2-methyl-1-(4-methylsulfanylphenyl)-2-morpholin-4-ylpropan-1-one |
| Molecular Structure | 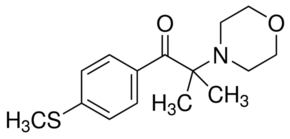 |
| CAS Registry Number | 71868-10-5 |
| EINECS Number | 400-600-6 |
| MDL Number | MFCD00083014 |
| Synonyms | 2-methyl-1-[4-(methylthio)phenyl]-2-morpholinopropan-1-one, 2-methyl-1-[4-(methylthio)phenyl]-2-(morpholin-4-yl)propan-1-one, 2-methyl-1-[4-(methylthio)phenyl]-2-(4-morpholinyl)-1-propanone, 2-methyl-2-(4-morpholinyl)-1-[4-(methylthio)phenyl]-1-propanone, 2-methyl-1-[4-(methylthio)phenyl]-2-morpholinyl-1-propanone, 2-methyl-1-(4-methylthiophenyl)-2-morpholino-propan-1-one, 2-methyl-1-[4-(methylthio)phenyl]-2-morpholinopropanone-1 |
| Molecular Formula | C15H21NO2S |
| Molecular Weight | 279.40 |
| InChI | InChI=1S/C15H21NO2S/c1-15(2,16-8-10-18-11-9-16)14(17)12-4-6-13(19-3)7-5-12/h4-7H,8-11H2,1-3H3 |
| InChI Key | LWRBVKNFOYUCNP-UHFFFAOYSA-N |
| Canonical SMILES | CC(C)(C(=O)C1=CC=C(C=C1)SC)N2CCOCC2 |
| Patent information | Title | Publication Date |
| CN109320476 | A α – amino acetyl thiophenol preparation method (by machine translation) | 2019 |
| CN109897015 | A class of free radical and cation hybrid LED initiator and its preparation method (by machine translation) | 2019 |
| US5026625 | Titanocenes, the use thereof, and n-substituted fluoroanilines | 1991 |
| US5192642 | Oxygen-containing titanocenes, and the use thereof | 1993 |
| US4992547 | Aminoaryl ketone photoinitiators | 1991 |
| US5008302 | Titanocenes, the use thereof, and N-substituted pyrroles | 1991 |
| US5068371 | Novel nitrogen-containing titanocenes, and the use thereof | 1991 |
Physical Data
| Appearance | White powder |
| Solubility | No data available |
| Flash Point | 165℃ |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C |
| 68 – 71 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 210 | 76 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz | Original Text (NMR Spectroscopy) |
| Chemical shifts | 1H | chloroform-d1 | 400.1 | 1H-NMR (400.1 MHz, CDCI3): = 1 .31 (5, 6H), 2.53 (5, 3H), 2.55-2.61 (m, 4H), 3.66-3.73 (m, 4H), 7.20-7.26 (m, 2H), 8.49-8.54 (m, 2 H) ppm. |
| Chemical shifts | 13C | chloroform-d1 | 100.6 | 13C-NMR (100.6 MHz, CDCI3): = = 14.4, 20.4, 47.2, 67.3, 68.3, 124.3, 130.3, 131.8, 144.9, 201.9 ppm. |
| Chemical shifts | 1H | chloroform-d1 | 400.1 | 1H-NMR (400.1 MHz, CDCIs): δ = 1 .31 (s, 6H), 2.53 (s, 3H), 2.55-2.61 (m, 4H), 3.66- 3.73 (m, 4H), 7.20-7.26 (m, 2H), 8.49-8.54 (m, 2 H) ppm. |
| Chemical shifts | chloroform-d1 | 100.6 | 13C-NMR (100.6 MHz, CDCIs): δ = 14.4, 20.4, 47.2, 67.3, 68.3, 124.3, 130.3, 131 .8, 144.9, 201 .9 ppm. | |
| 1H | chloroform-d1 | 300 | 1 H-NMR (300MHz, CDCI3), S [ppm]: 1.31 (s, 6H), 2.53 (s, 3H), 2.56-2.59 (m, 4H), 3.68-3.71 (m, 4H), 7.21-7.24 (m, d-like, 2H), 8.50-8.53 (m, d-like, 2H). |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | |||
| Spectrum | acetonitrile | ||
| Band assignment, Spectrum | benzene | 370 | |
| methanol | 366 | 121 | |
| N,N-dimethyl-formamide | 230, 305 | ||
| Spectrum | acetonitrile |
Route of Synthesis (ROS)
| Conditions | Yield |
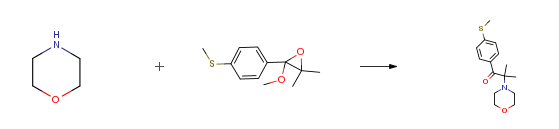
| With calcium hydroxide at 20 – 138℃; for 20h; Experimental Procedure 241.8 g of the epoxide, prepared in 8.2, are dissolved in 223 g of morpholine in a 1 L reactor at room temperature and mixed with 2.7 g of calcium hydroxide. The reaction mixture is heated to 125-138°C and the resulting methanol is continuously distilled off over a period of 2Oh. Afterwards the residual morpholine is evaporated yielding 294.4 g of the crude α- aminoketone photo initiator, which is crystallized from methanol giving 248.8 g (90.2 percent) 2- methyl-1-[4-(methylthio)phenyl]-2-(4-morpholinyl)-1-propanone. | 90.2% |
| With tetra(n-butyl)ammonium hydrogensulfate; sodium hydroxide for 8h; Reflux; Experimental Procedure Example 2: Preparation of 2-methyl-1 -(4-methylthiophenyl)-2-morpholino-propan- 1 -one (0247) A mixture of 2-methoxy-3,3-dimethyl-2-(4-methylthiophenyl)oxirane (0.13 g, 0.58 mmol), morpholine (2.27 g, 26.1 mmol), sodium hydroxide solution (50percent, 0.28 g, 3.5 mmol) and tetrabutylammonium hydrogensulfate (0.01 g, 0.03 mmol) was stirred under reflux for 8 h. The reaction mixture was cooled down to room temperature, the solvent was evaporated under reduced pressure and the residue was taken up with water (10 mL). The phases were separated and the pH of the aqueous layer was adjusted to 7 with saturated ammonium chloride solution. The aqueous layer was extracted with ethyl acetate (3 x 5 mL). The combined organic layers were washed with brine (10 mL), dried over Na2SO4 and the solvent was evaporated. 2-methyl-1 -(4-methylthiophenyl)-2- morpholino-propan-1 -one was obtained as a colorless oil (70 mg, 0.25 mmol, 43 percent yield). (0248) 1H-NMR (400.1 MHz, CDCIs): δ = 1 .31 (s, 6H), 2.53 (s, 3H), 2.55-2.61 (m, 4H), 3.66- 3.73 (m, 4H), 7.20-7.26 (m, 2H), 8.49-8.54 (m, 2 H) ppm. 13C-NMR (100.6 MHz, CDCIs): δ = 14.4, 20.4, 47.2, 67.3, 68.3, 124.3, 130.3, 131 .8, 144.9, 201 .9 ppm. | 43% |
| at 105 – 110℃; for 20h; Temperature; | 119.3g |
| at 105 – 115℃; for 20h; | 112.57g |
Safety and Hazards
| Pictogram(s) |    |
| Signal | Danger |
| GHS Hazard Statements | H302: Harmful if swallowed [Warning Acute toxicity, oral] H360FD: May damage fertility; May damage the unborn child [Danger Reproductive toxicity] H411: Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] |
| Precautionary Statement Codes | P201, P202, P264, P270, P273, P281, P301+P312, P308+P313, P330, P391, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 9; Packaging Group: III; UN Number: 3077 |
| Under the room temperature and away from light | |
| HS Code | 291439 |
| Storage | Under the room temperature and away from light |
| Shelf Life | No data available |
| Market Price | USD |
| Use Pattern |
| 2-Methyl-4′-(methylthio)-2-morpholinopropiophenone CAS 71868-10-5 is as a photoinitiator |
| Component of photosensitive composition |
| 2-Methyl-4′-(methylthio)-2-morpholinopropiophenone CAS 71868-10-5 is as photoinitiator for polymerisation |
| 2-Methyl-4′-(methylthio)-2-morpholinopropiophenone CAS 71868-10-5 is as Curing initiator |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |