2,2′-DIPICOLYLAMINE CAS#: 1539-42-0; ChemWhat Code: 4536
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| KR2018/11949 | Novel 2-amine substituted 1,4-naphthoquinone compounds and pharmaceutical composition for preventing or treating cancer comprising the same as an active ingredient | 2018 |
| US2014/212335 | MAGNETIC SEPARATION USING NANOPARTICLES | 2014 |
| US5708022 | Method for inhibiting immune response | 1998 |
| EP2030971 | PEST CONTROL AGENT CONTAINING NOVEL PYRIDYL-METHANAMINE DERIVATIVE OR SALT THEREOF | 2009 |
| WO2008/122837 | COMPOSITIONS AND METHODS FOR CONTROLLING INFESTATION | 2008 |
| WO2006/11719 | N-SUBSTITUTED-SULFAMOYLBENZOIC ACID DERIVATIVES, METHOD FOR PREPARING THEREOF AND ANTIVIRAL PHARMACEUTICAL COMPOSITION COMPRISING THE SAME | 2006 |
| US2003/235843 | Technetium-depyridine complexes, and methods of use thereof | 2003 |
Physical Data
| Appearance | Yellow to light yellow transparent liquid |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 107 – 110 | 0.0675068 |
| 130 – 135 | 0.1 |
| 130 – 145 | 0.1 |
| 125 | 0.1 |
| 146 – 149 | 0.5 |
| 148 | 1 |
| 148 – 149 | 1.05 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C |
| Enthalpy of association | aq. buffer | 25 |
| Association with compound | aq. buffer | |
| Enthalpy of association | aq. buffer | 25 |
| Association with compound | aq. buffer | |
| Association with compound | water | 20 |
| NMR spectrum of the complex | dimethylsulfoxide-d6 | 24.85 |
| Stability constant of the complex with … | dimethylsulfoxide | 24.85 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) |
| Chemical shifts | 1H | chloroform-d1 |
| Chemical shifts | 13C | chloroform-d1 |
| Chemical shifts | 1H | chloroform-d1 |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 |
| Chemical shifts, Spectrum | 13C | dimethylsulfoxide-d6 |
| Chemical shifts | 1H | chloroform-d1 |
| Chemical shifts, Spectrum | 1H | chloroform-d1 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | potassium bromide |
| Bands | potassium bromide |
| ATR (attenuated total reflectance), Bands, Spectrum | |
| Intensity of IR bands, Bands, Spectrum | potassium bromide |
| Bands | neat (no solvent), sodium chloride |
| ATR (attenuated total reflectance), Bands, Spectrum | |
| Spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | ||
| Spectrum | 205, 261, 351 | |
| Spectrum | acetonitrile | |
| Spectrum | methanol | |
| Band assignment, Spectrum | acetonitrile |
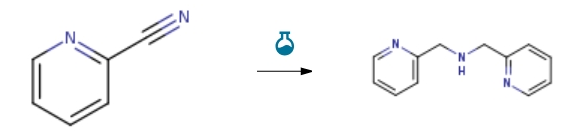
Route of Synthesis (ROS)
| Conditions | Yield |
| With palladium-carbon; hydrogen In ethanol for 72h; Molecular sieve; Experimental Procedure 3 Example 3: Preparation of 2,2’-Dipicolylamine (DPA) To a round bottom flask equipped with a magnetic stir bar was added; 10.1015 g 2-cyanopyridine [96.05 mmol], 0.4966g 5% (wt/wt) Palladium on Carbon and 16.5 mL of anhydrous ethanol stored over 4 A molecular sieves. The reaction mixture was then slowly flushed with 3 L of H2(g) and once flushed, a vacuum was pulled on the reaction mixture before a balloon of hydrogen 1 L was placed on top to incorporate overnight. The reaction vessel was flushed with a balloon of fresh hydrogen every day. Reaction progress was monitored by TLC [Eluent – 20% CH3OH: 80% CH2C12] and visualized with acidic ninhydrin stain. The reaction occurred over 72 hours, after which TLC indicated the consumption of starting material. 1001511 Isolation of 2,2 ‘-Dipicolylamine: The reaction mixture was filtered through a 1 cmthick layer of packed celite by vacuum filtration. The celite was then washed with 50 mL of hotethanol and the collected filtrates were combined and concentrated by rotary evaporation. Theafforded 9.540 g [99.7% isolated] of transparent amber oil was verified by ESI-LCMS [M/Z+1200.1 positive ionization – IVJIZ-1 : 198.95 negative ionization] and 1H NMR.1001521 1HNMR 300 MHz [CD3C1]: 3.97, s,H4; 7.21,t(Jc. 6.9Hz),H2; 7.38, d(Jc. 8.7Hz), H 2; 7.64, td (Jc. 6.9 Hz) H 2; 8.55, d (Jc. 7.2 Hz) H 2. | 99.7% |
| With sodium tetrahydroborate; La0.5Ca0.5CoO3 In methanol at 40℃; under 760.051 Torr; for 0.583333h; chemoselective reaction; Experimental Procedure General procedure for the reduction of nitriles into their correspondingsec-amines. General procedure: To a stirring mixture of the appropriate nitrile (1 mmol) and nanosized La0.5Ca0.5CoO3 perovskite (1 mol%, 1.9 mg) in MeOH (5 ml) at 40C, NaBH4 (2 mmol, 0.08 g) was added portion wise. After the completion of the reaction which was monitored by TLC using CHCl3: MeOH (30:1) as eluent, the catalyst was centrifuged off and the solvent was evaporatedunder reduced pressure. To the resulting oily liquid, water (20 ml) was added and the mixture was extracted with CH2Cl2 (2 × 20 ml). The organic layer was dried over anhydrous Na2SO4 and the solvent was evaporated under reduced pressure to afford the pure products. | 85% |
| With hydrogen; palladium-on-charcoal In ethanol; water for 9h; Ambient temperature; |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H301 (29.51%): Toxic if swallowed [Danger Acute toxicity, oral] H310 (29.51%): Fatal in contact with skin [Danger Acute toxicity, dermal] H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P262, P264, P264+P265, P270, P271, P280, P301+P316, P302+P352, P304+P340, P305+P351+P338, P316, P319, P321, P330, P332+P317, P337+P317, P361+P364, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| HS Code | |
| Storage | Store at 2~8°C and away from light for long time. |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 199.255 |
| logP | -0.918 |
| HBA | 3 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 37.81 |
| Rotatable Bond (RotB) | 4 |
| Matching Veber Rules | 2 |
| Quantitative Results | ||
| 1 of 134 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Method for inhibiting immune response | |
| 2 of 134 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | The kinetics of interaction of nickel(II) and cobalt(II) with bis(2-pyridylmethyl)amine | |
| 3 of 134 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | An Iron(III) Complex with 2-<Bis(2-pyridylmethyl)aminomethyl>-4-nitrophenol as an Intradiol Dioxygenase Model Compound | |
| 4 of 134 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Alkylaluminum complexes containing pyridyl amido ligands. Syntheses and characterization of AlMe<sub>2</sub>[N(CH<sub>2</sub>-2-Py)<sub>2</sub>], Al<sub>2</sub>Me<sub>5</sub>[N(CH<sub>2</sub>-2-Py)<sub>2</sub>], and Al<sub>2</sub>Me<sub>4</sub>[2,3,5,6-tetra(2-pyridyl)piperazyl], an unusual carbon-carbon bond coupling product | |
| 5 of 134 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | A model for Zn(II)-containing-β-lactamase: Synthesis, X-ray crystal structure of a Zinc(II) complex bearing thiol group and hydrolysis of phosphate diester | |
| 6 of 134 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | First artificial receptors and chemosensors toward phosphorylated peptide in aqueous solution | |
| 7 of 134 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | A novel two-fluorophore approach to ratiometric sensing of Zn<sup>2+</sup> | |
| 8 of 134 | Comment (Pharmacological Data) | Comment (Pharmacological Data) |
| Reference | Multiple logical access with a single fluorophore-spacer-receptor system: Realization of inhibit (INH) logic function |
| Use Pattern |
| Bis(2-pyridinylmethyl)amine CAS 1539-42-0 is used in various applications, including as a ligand in coordination chemistry, where it can form complexes with metal ions. It may also be used as an intermediate in organic synthesis, including pharmaceuticals and other fine chemicals. 1. As an intermediate, it is used to synthesize compounds such as terpenes, steroids, sugars and nucleosides. 2. As a pharmaceutical intermediate, it may participate in the synthesis of a variety of drugs. 3. Pesticide, fragrance, dye, pigment synthesis: in these fields, dimethylpyridinamine can be used as a synthetic intermediate or catalyst. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |