2,3,6,7,10,11-Triphenylenehexol CAS#: 4877-80-9; ChemWhat Code: 1411199
Identification
| Product Name | 2,3,6,7,10,11-Triphenylenehexol |
| IUPAC Name | triphenylene-2,3,6,7,10,11-hexol |
| Molecular Structure | 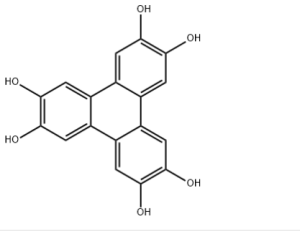 |
| CAS Registry Number | 4877-80-9 |
| EINECS Number | 207-322-2 |
| MDL Number | MFCD01321170 |
| Beilstein Registry Number | |
| Synonyms | 4877-80-9 2,3,6,7,10,11-Hexahydroxytriphenylene Triphenylene-2,3,6,7,10,11-hexaol Triphenylene-2,3,6,7,10,11-hexol 2,3,6,7,10,11-Triphenylenehexol MFCD01321170 YSZC282 SCHEMBL212835 DTXSID10454818 AMY38445 CX1151 ZINC38229869 AKOS016003185 CS-W013943 AS-10440 SY069448 2,3,6,7,10,11-hexa-hydroxytriphenylene DB-005824 FT-0650928 H0907 2,3,6,7,10,11-Hexahydroxytriphenylene, Hydrate A871880 |
| Molecular Formula | C18H12O6 |
| Molecular Weight | 324.28 |
| InChI | InChI=1S/C18H12O6/c19-13-1-7-8(2-14(13)20)10-4-17(23)18(24)6-12(10)11-5-16(22)15(21)3-9(7)11/h1-6,19-24H |
| InChI Key | QMLILIIMKSKLES-UHFFFAOYSA-N |
| Canonical SMILES | C1=C2C3=CC(=C(C=C3C4=CC(=C(C=C4C2=CC(=C1O)O)O)O)O)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| JP5731346 | Method of manufacturing tripenylene compd. | 2015 |
| JP2015/189756 | METHOD OF PRODUCING POLYHYDRIC PHENOL COMPOUND | 2015 |
| EP2177495 | PROCESS FOR PRODUCING TRIPHENYLENE COMPOUND AND CRYSTAL OBTAINED BY THE PROCESS | 2010 |
Physical Data
| Appearance |
| Melting Point, °C | Solvent (Melting Point) |
| 387 | various solvent(s) |
| Boiling Point, °C |
| 251 |
| 250 – 252 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
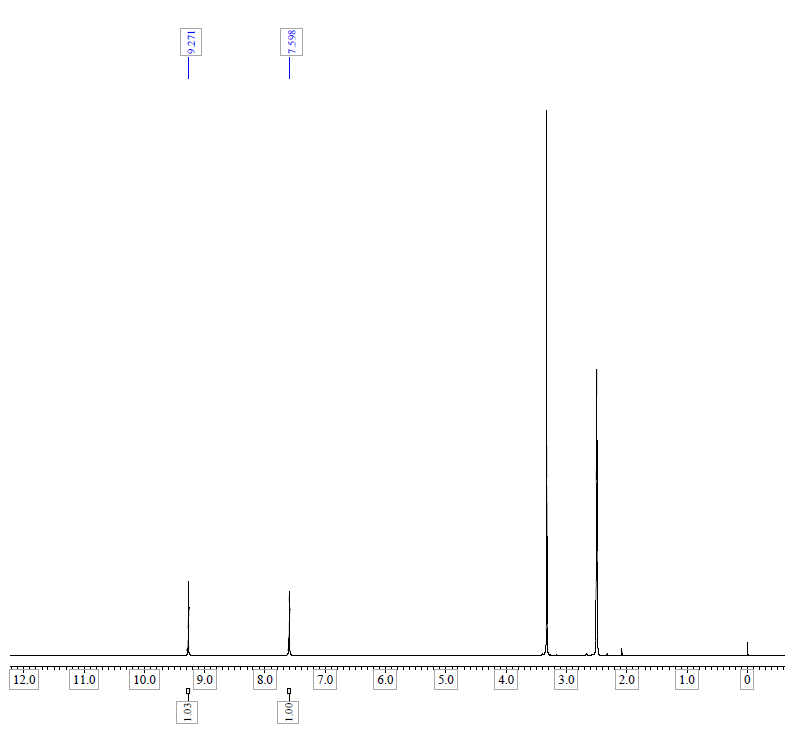
| Chemical shifts, Spectrum | 1H | chloroform-d1 | ||
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 400 | |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 500 | |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | 500 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands, Spectrum | ||
| Spectrum | potassium bromide | |
| Bands, Spectrum | potassium bromide |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | 254 | |||
| 346 | 40000 | |||
| Spectrum |
Route of Synthesis (ROS)
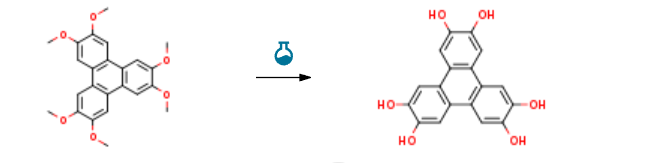
Route of Synthesis (ROS) of 2,3,6,7,10,11-Triphenylenehexol CAS-4877-80-9
| Conditions | Yield |
| With boron tribromide In dichloromethane at -70 – 20℃; | 100% |
| With boron tribromide In dichloromethane at 20℃; | 100% |
| With boron tribromide In dichloromethane at -70 – 20℃; Inert atmosphere; | 98% |
| Experimental Procedure 2.3.4. Synthesis 2,3,6,7,10,11-hexahydroxytriphenylene (4) The preparation of compound 4 is similar to that reported in the literature[14]. Hexamethoxytriphenylene (7 g, 17.14 mmol) was dissolved in dichoromethane (50 ml) and the solution thus obtained was cooled in ice bath. A solution of boron tribromide (11.47 ml) was then added slowly to the reaction mixture over a period of 30 min. After complete addition, the reaction was left stirring overnight in room temperature.The reaction mixture was then slowly poured into crushed ice(100 g) and the obtained mixture was stirred vigorously until the ice melted. It was then extracted with ethyl acetate (6 × 150 ml), dried over anhydrous sodium sulphate and evaporated to dryness giving dark purple solid. The yield was quantitative and this compound was used without further purification.erimental Procedure 2.3.4. Synthesis 2,3,6,7,10,11-hexahydroxytriphenylene (4) The preparation of compound 4 is similar to that reported in the literature[14]. Hexamethoxytriphenylene (7 g, 17.14 mmol) was dissolved in dichoromethane (50 ml) and the solution thus obtained was cooled in ice bath. A solution of boron tribromide (11.47 ml) was then added slowly to the reaction mixture over a period of 30 min. After complete addition, the reaction was left stirring overnight in room temperature.The reaction mixture was then slowly poured into crushed ice(100 g) and the obtained mixture was stirred vigorously until the ice melted. It was then extracted with ethyl acetate (6 × 150 ml), dried over anhydrous sodium sulphate and evaporated to dryness giving dark purple solid. The yield was quantitative and this compound was used without further purification.erimental Procedure General procedure: In a typical experiment, 0.5mmol of nitroarene and 0.002g(2mol%) NiNPs/DNA were added to 2mL water and thenstirred for 2-3min for thoroughly mixing. Subsequently,1mmol of NaBH4was added to the reaction mixture undermagnetic stirring at room temperature. The extent of thereaction was monitored by thin layer chromatography.Reproducibility of the results was checked by repeating theruns at least three times and was found to be within acceptablelimits (± 3%). When the reaction was completed, thereaction mixture was diluted with ethyl acetate and the catalystwas recovered by centrifugation. The combined organicfractions were dried over Na2SO4and evaporated underreduced pressure. The crude product was purified by columnchromatography on silica gel with a mixture of ethyl acetateand n-hexane as the eluent, and the ratio of ethyl acetate andn-hexane was depended on the structure of the products.The structure of isolated products was verified by 1H NMR. | 97% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (50%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 6.1; Packaging Group: II; UN Number: 2671 |
| Under the room temperature and away from light | |
| HS Code | 290621 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD 45/kg |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 324.29 |
| logP | 4.403 |
| HBA | 0 |
| HBD | 6 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 121.38 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Quantitative Results | ||
| 1 of 24 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | ||
| 2 of 24 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | ||
| 3 of 24 | Comment (Pharmacological Data) | Bioactivities present |
| Reference |
| Use Pattern |
| 2,3,6,7,10,11-Triphenylenehexol CAS#: 4877-80-9 is used as a new COF/MOF Ligand. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |