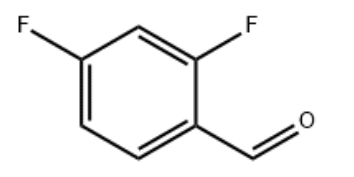
2,4-Difluorobenzaldehyde(DFBA) CAS#: 1550-35-2; ChemWhat Code: 1411400
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN114105971 | 6-(benzo 1, 3 dioxopentacyclic)-4phenyl-6H-1, 3-thiazine-2-amine derivative and application | 2022 |
| CN113024414 | Method for efficiently synthesizing fluorine-containing compound | 2021 |
| CN110590665 | Camphoric acid-based acylhydrazone derivative, preparation method and applications thereof | 2019 |
| WO2016/149311 | FUNGICIDAL PYRAZOLES | 2016 |
Physical Data
| Appearance | Colorless to pale yellow transparent liquid |
| Melting Point, °C | Solvent (Melting Point) |
| 2 – 3 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 80 – 90 | 30 – 35 |
| 82 | 42 |
| 50 – 52 | 16 |
| 62 – 63 | 11 |
| 65 – 66 | 17 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.14 | 4 | 25 |
| 1.2 | 4 | -190 |
| 1.24 |
Spectra
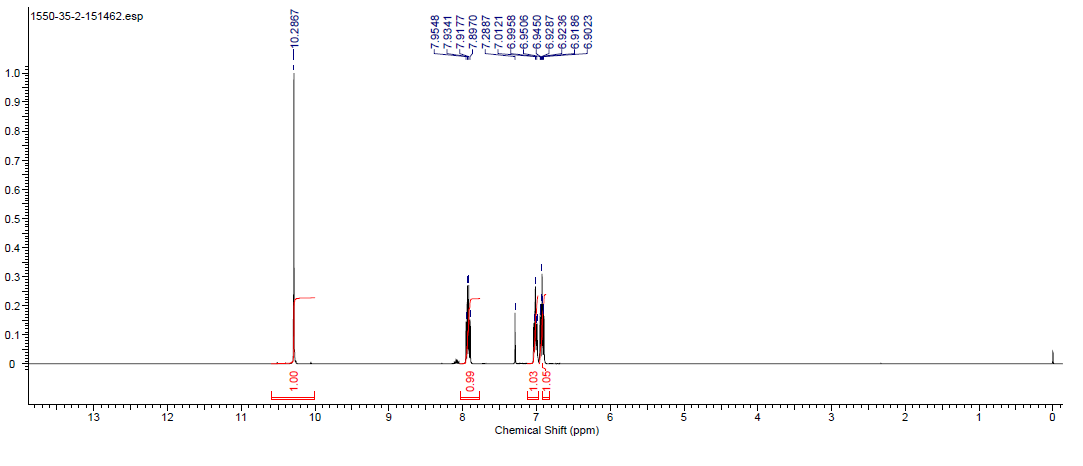
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | 400 | |
| Chemical shifts | 13C | chloroform-d1 | 100 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 | |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 101 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands | potassium bromide | |
| Bands | potassium bromide | |
| FT-IR-Difference Spectroscopy, Bands, Spectrum | ||
| Bands, Spectrum | ||
| Bands | ||
| Bands | nujol |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) |
| Spectrum | gas | 220 – 250 nm |
Route of Synthesis (ROS)
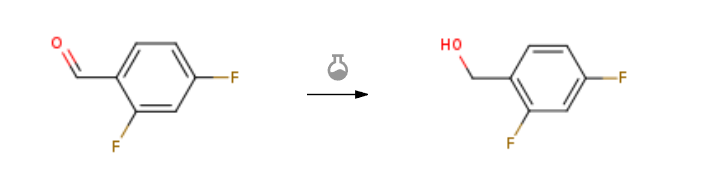
Route of Synthesis (ROS) of 2,4-Difluorobenzaldehyde(DFBA) CAS1550-35-2
| Conditions | Yield |
| With sodium tetrahydroborate In methanol at 0℃; Inert atmosphere; | 100% |
| Stage #1: 2,4-difluorobenzaldehyde With C36H44F4N2Ni2P2; diphenylsilane In tetrahydrofuran at 45℃; for 3h; Schlenk technique; Inert atmosphere; Stage #2: With sodium hydroxide In tetrahydrofuran; methanol at 60℃; for 24h; Schlenk technique; Inert atmosphere; | 92% |
| With methanol; sodium tetrahydroborate at 0℃; for 1h; | 88% |
| Experimental Procedure Example 13; Preparation of Intermediates (1-(Bromomethyl)-2,4-difluorobenzene) (I-1)To a stirred solution of 2,4-difluorobenzaldehyde (500 mg, 3.52 mmol) in CH3OH (8 mL) was added NaBH4 (266 mg, 7.04 mmol) portion wise at 0° C., and the reaction mixture was stirred at 0° C. for 1 h. After completion of the reaction (by TLC), CH3OH was removed under reduced pressure, diluted with ice-cold H2O (40 mL) and extracted with EtOAc (2×20 mL). The combined organic layers were washed with H2O (40 mL) and brine (40 mL), dried over anhydrous Na2SO4 and concentrated under reduced pressure to obtain the crude material. Purification by silica gel column chromatography eluting with 10% EtOAc/hexanes afforded the alcohol G (450 mg, 3.12 mmol, 88%) as colorless liquid. 1H NMR (200 MHz, CDCl3): δ 7.45-7.33 (m, 1H), 6.83-6.75 (m, 2H), 4.72 (s, 2H), 1.79 (br s, OH). |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H300: Fatal if swallowed [Danger Acute toxicity, oral] H301: Toxic if swallowed [Danger Acute toxicity, oral] H311: Toxic in contact with skin [Danger Acute toxicity, dermal] H315: Causes skin irritation [Warning Skin corrosion/irritation] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H331: Toxic if inhaled [Danger Acute toxicity, inhalation] H335: May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] H373: Causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] H400: Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410: Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P260, P261, P264, P270, P271, P273, P280, P301+P310, P302+P352, P304+P340, P305+P351+P338, P311, P312, P314, P321, P322, P330, P332+P313, P337+P313, P361, P362, P363, P391, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Do not expose to air. Store under an inert atmosphere |
| HS Code | |
| Storage | Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Do not expose to air. Store under an inert atmosphere |
| Shelf Life | 1 year |
| Market Price | USD 300/kg |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 142.105 |
| logP | 2.04 |
| HBA | 1 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 17.07 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Quantitative Results | ||
| 1 of 59 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | 4-HYDROXYPIPERIDINE DERIVATIVES HAVING ANTIARRHYTHMIC EFFECT | |
| 2 of 59 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Triazolo-pyridazine derivatives as ligands for GABA receptors | |
| 3 of 59 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | ||
| 4 of 59 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Synthesis and calcium antagonistic activity of diethyl styrylbenzylphosphonates | |
| 5 of 59 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Synthesis and antitumor activities of novel 5-deazaflavin-sialic acid conjugate molecules | |
| 6 of 59 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | 3-Trifluoromethyl-4-nitro-5-arylpyrazoles are novel KATP channel agonists | |
| 7 of 10 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Cross reactions of cyanoacetic acid derivatives with carbonyl compounds 3. One-step synthesis of substituted 2-amino-5-oxo-4,5-dihydropyrano[3,2-c] chromenes |
| Use Pattern |
| 2,4-Difluorobenzaldehyde(DFBA) CAS#: 1550-35-2 isused in medicine, pesticide, liquid crystal material intermediates. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watsonnoke Scientific Limited | https://www.watsonnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |