2,6-Difluorobenzonitrile CAS#: 1897-52-5; ChemWhat Code: 78931
Identification
| Product Name | 2,6-Difluorobenzonitrile |
| IUPAC Name | 2,6-difluorobenzonitrile |
| Molecular Structure | 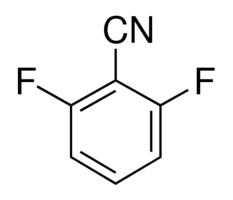 |
| CAS Registry Number | 1897-52-5 |
| EINECS Number | 217-589-7 |
| MDL Number | MFCD00001778 |
| Beilstein Registry Number | 2045292 |
| Synonyms | 3-Pyridinamin;3-Pyridinamine;3-Pyridinamine;pyridin-3-amine;T6NJ CZ;3- Aminopyridine;3-Amino-pyridine;3-pyridylamine;Amino-3 pyridine;m-Aminopyridine;MS/MS-1064463;Pyridin-3-ylamine;Pyridine, 3-amino-;β-Aminopyridine 462-08-8 |
| Molecular Formula | C7H3F2N |
| Molecular Weight | 139.102 |
| InChI | InChI=1S/C7H3F2N/c8-6-2-1-3-7(9)5(6)4-10/h1-3H |
| InChI Key | BNBRIFIJRKJGEI-UHFFFAOYSA-N |
| Canonical SMILES | c1cc(c(c(c1)F)C#N)F |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2008/152089 | NOVEL COMPOUNDS | 2008 |
| EP1248313 | Polymer electrolyte and process for producing the same | 2002 |
| US2006/68467 | Rhodococcus nitrile hydratase | 2006 |
| JP2005/112745 | Method for producing aromatic fluorine compound | 2005 |
| US6392084 | Method for production of organic fluorine compound | 2002 |
Physical Data
| Appearance | Colorless solid or oily liquid |
| Boiling point | 197-198℃ |
| Melting Point, °C | Solvent (Melting Point) |
| 28 – 29 | benzene |
| 30 – 31 | |
| 29 – 30 | petroleum ether |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 81 – 82 | 15 |
| 81 – 83 | 12 |
| 98 – 100 | 31 |
| 81 – 83 | 13 |
| 99 | 20 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 300 | |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 125 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 22 | 300 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 22 | 75 |
| Chemical shifts | 19F | CDCl3 | ||
| Chemical shifts | 19F | dimethylsulfoxide-d6 | ||
| Chemical shifts | 19F | dimethylsulfoxide-d6 | ||
| NMR | ||||
| Spin-spin coupling constants |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | KBr |
| Bands | nujol |
| Bands | gas |
| Bands | neat (no solvent) |
| Description (Mass Spectrometry) |
| spectrum |
| high resolution mass spectrometry (HRMS), electrospray ionisation (ESI), time-of-flight mass spectra (TOFMS), spectrum |
| electron impact (EI), spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | 200 – 500 nm | ||
| Spectrum | not given | 255.2 – 292.3 nm | |
| Absorption maxima | 268, 213.6, 211.8 | ||
| Absorption maxima | not given | Ratio of solvents: 0.1N | 282.7, 277.7, 274.7, 220.3 |
| Spectrum | gas | 200 – 250 nm |
Route of Synthesis (ROS)
| Conditions | Yield |
| With ammonia In dimethyl sulfoxide at 80℃; for 80h; closed bottle; | 99% |
| With ammonia In ethanol at 140℃; under 10343.2 Torr; for 6h; Experimental Procedure 10 [ EXAMPLE 10] 3, 2′-Difluoro-5′-[8-fluoro-7-(1-hydroxy-1-methyl)imidazo[1, 2- [AL-PVRIDIN-3-YLLBIL)] biphenyl-2-carbonitrile A mixture [OF 2, 6-DIFLUOROBENZONITRILE] (19.0 g, 137 mmol) and ethanol (200 ml) pre-saturated with ammonia gas was heated at [140°C] in an autoclave for 6 h (terminal pressure 200 psi). The mixture was allowed to cool to ambient temperature, evaporated to dryness and triturated with water (200 ml). The solid was filtered and left to air-dry to afford 2-amino- 6-fluorobenzonitrile (18.0 g, 97%) as an off-white solid: [8H] (360 MHz, [CDCL3)] 4.53 (3H, s), 6.44-6. 52 (2H, m), 7.24-7. 30 [(1H, M).] 2-Amino-6-fluorobenzonitrile (18.0 g, 132 mmol) was dissolved in hot 1,4-dioxane (20 ml), 48% hydrobromic acid (200 [ML)] was added and the mixture cooled to [0°C] before dropwise addition of sodium nitrite (10.5 g, 152 mmol) in water (20 [ML)] over 1.5 h. The resulting mixture was stirred at 0°C for 1.5 h then poured onto a cooled [(0°C)] solution of copper [(I)] bromide (56.8 g, 396 mmol) in 48% hydrobromic acid (50 ml). The solution was stirred at [0°C] for 15 min then heated at [50°C] for 20 min. The mixture was cooled to ambient temperature, diluted with water (1200 [ML)] and extracted with ethyl acetate (2 x 400 [ML).] The combined organics were washed with [10%] aqueous ammonia solution (400 ml), water (400 ml) and brine (500 ml), dried over anhydrous magnesium sulphate, filtered and evaporated to give an orange oil. Purification by chromatography on silica gel, eluting with isohexane on a gradient of ethyl acetate (2-4%), gave 2- bromo-6-fluorobenzonitrile (18.5 g, 70%) as a white solid: [8H] (400 MHz, [CDCL3)] 7.17-7. 23 [(1H,] ddd, [J8, 8] and 1), 7.44-7. 52 (2H, m). [2-BROMO-6-FLUOROBENZONITRILE] and [2- (2-FLUORO-5-NITROPHENYL)-4,] 4,5, 5- tetramethyl- [1, 3, [2]] dioxaborolane were coupled following the procedure in Example 2 to afford 3, 2′-difluoro-5′-nitrobiphenyl-2-carbonitrile as a black solid: [5H] (360 MHz, [CDCL3)] 7.32-7. 44 (3H, m), 7.71-7. 77 [(1H,] m), 8. 35-8. 41 (2H, m). 3, [2′-DIFLUORO-5′-NITROBIPHENYL-2-CARBONITRILE] was reduced following the procedure in Example 2 to give 5′-amino-3,2′-difluorobiphenyl-2- carbonitrile as a brown solid: [SN] (360 MHz, [CDCL3)] 3.74 (2H, s), 6.66-6. 75 (2H, m), 7.01 [(1H,] dd, J 9 and 9), 7.19-7. 30 (2H, m), 7.59-7. 65 [(1H,] m). 5′-Amino-3, 2′-difluorobiphenyl-2-carbonitrile was bromo-de- aminated following the procedure in Example 2 to give 5′-bromo-3,2′- difluorobiphenyl-2-carbonitrile as a white solid: AH (400 MHz, [CDCL3)] 7.13 [(1H,] dd, J 9 and 9), 7.27-7. 30 (2H, m), 7.53-7. 59 (2H, m), 7.64-7. 69 [(1H,] m). [5′-BROMO-3,] [2′-DIFLUOROBIPHENYL-2-CARBONITRILE WAS] converted, following the procedure in Example 2, to 3, [2′-DIFLUORO-5′- (5,] 5-dimethyl- [1, 3, 2] dioxaborinan-2-yl) biphenyl-2-carbonitrile, a brown oil that crystallised on standing: [SN] (360 MHz, [CDCL3)] 1.03 (6H, s), 3.77 (4H, s), 7.17-7. 25 (2H, m), 7.30 [(1H,] d, J8), [7.] 59-7.65 [(1H,] m), 7.81-7. 91 (2H, m). 3-Bromo-8-fluoro-7- [(1-HYDROXY-1-METHYLETHYL)] imidazo [1, 2-a] pyridine (0.10 g, 0.36 mmol) and 3, [2′-DIFLUORO-5′- (5, 5-DIMETHYL- [1,] 3,2] [DIOXABORINAN-] 2-yl) biphenyl-2-carbonitrile (0.16 g, 0.47 mmol) were coupled following the procedure in Example 1 to afford [3, 2′-DIFLUORO-5′- [8-FLUORO-7- (L-HYDROXY-L-] methylethyl) imidazo [1, 2-a] pyridin-3-yl] biphenyl-2-carbonitrile as a white amorphous solid (110 mg, 74%): [8H] (360 MHz, [CDCL3)] 1.73 (6H, s), 2.10 [(1H,] s), 7.21 [(1H,] dd, J 7 and 7), 7.30 [(1H,] dd, J 8 and [8), 7. 38-7.] 44 (2H, m), 7.61-7. 72 (4H, m), 8. 23 | 97% |
| With ammonia In dimethyl sulfoxide at 75 – 90℃; for 24h; | 90% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302 (97.94%): Harmful if swallowed [Warning Acute toxicity, oral] H312 (81.44%): Harmful in contact with skin [Warning Acute toxicity, dermal] H315 (48.45%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (48.45%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H332 (80.41%): Harmful if inhaled [Warning Acute toxicity, inhalation] H335 (46.39%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | 292690 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 139.104 |
| logP | 2.067 |
| HBA | 1 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 23.79 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Use Pattern |
| 2,6-Difluorobenzonitrile CAS#: 1897-52-5 as intermediates for the synthesis of agricultural chemicals, medicines, and dyes |
| 2,6-Difluorobenzonitrile CAS#: 1897-52-5 is a new type of pesticide intermediate, which is mainly used for the production of high-efficiency, low-toxicity and broad-spectrum fluorine-containing benzoylurea pesticides and herbicide intermediates 2,6-difluorobenzamide . |
| 2,6-Difluorobenzonitrile CAS#: 1897-52-5 is also used in medicine. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

