3-CHLORO-2-HYDROXYPROPANESULFONIC ACID SODIUM SALT CAS#: 126-83-0; ChemWhat Code: 37905
Identification
| Product Name | 3-CHLORO-2-HYDROXYPROPANESULFONIC ACID SODIUM SALT |
| IUPAC Name | sodium;3-chloro-2-hydroxypropane-1-sulfonate |
| Molecular Structure | 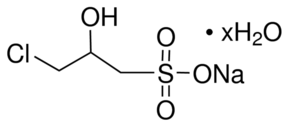 |
| CAS Registry Number | 126-83-0 |
| EINECS Number | 204-807-0 |
| MDL Number | MFCD00149699 |
| Beilstein Registry Number | No data available |
| Synonyms | sodium 3-chloro-2-hydroxypropane-sulfonate, sodium 1-chloro-2-hydroxy-3-propanesulfonate, 3-chloro-2-hydroxy-1-propanesulfonic acid sodium salt, 3-chloro-2-hydroxypropyl sulfonic acid sodium salt, monosodium 3-chloro-2-hydroxy-1-propanesulfonate, 3-chloro-2-hydroxypropanesulfonic acid sodium, sodium 3-chloro-2-hydroxypropane-1-sulfonate |
| Molecular Formula | ClCH2CH(OH)CH2SO3Na · xH2O |
| Molecular Weight | 196.59 |
| InChI | InChI=1S/C3H7ClO4S.Na/c4-1-3(5)2-9(6,7)8;/h3,5H,1-2H2,(H,6,7,8);/q;+1/p-1 |
| InChI Key | TZLNJNUWVOGZJU-UHFFFAOYSA-M |
| Canonical SMILES | C(C(CCl)O)S(=O)(=O)[O-].[Na+] |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2014/24855 | PROCESS TO PRODUCE FLUORINATED BETAINES | 2014 |
| US2013/267707 | N,N+hu 1 +l SUBSTITUTED PIPERAZINES HAVING COMBINED ANTIAGGREGANT, ANTICOAGULANT AND VASODILATORY ACTIVITY, AND METHOD FOR PRODUCING SAME | 2013 |
| WO2003/82811 | MANUFACTURING METHOD OF MONOGLYCERIDE SULFONATE, TOILET SOAP COMPOSITION USING THE SAME, AND MANUFACTURING METHOD OF TOILET SOAP COMPOSITION COMPRISING SALT | 2003 |
Physical Data
| Appearance | White crystalline powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C |
| 251 – 252.2 |
| 251.4 – 252.5 |
| 251.9 – 252.8 |
| 252.1 – 252.6 |
| 251.9 – 252.6 |
| 252 – 252.8 |
| 246 – 247 |
| Description (Association (MCS)) |
| H2O (g/100 g H2O), 20-90grad |
Spectra
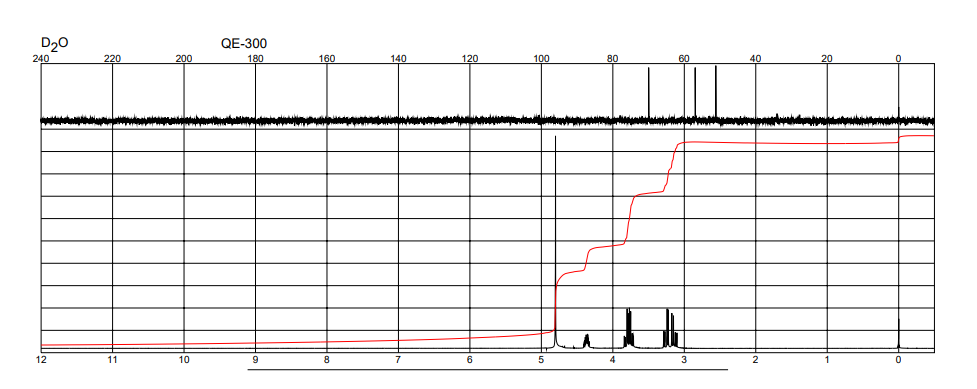
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) |
| Chemical shifts, Spectrum | 1H | water-d2 |
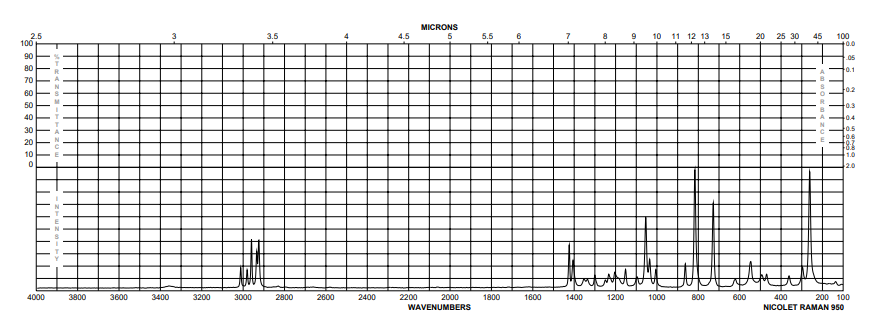
| Description (IR Spectroscopy) |
| Bands |
| Bands |
| Bands, Spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
| With sodium hydrogensulfite In water at 85℃; | 80.6% |
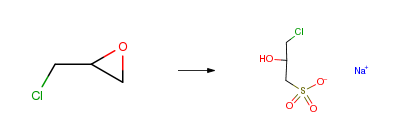
| With sodium hydrogensulfite In water Experimental Procedure A. Synthesis of 3-chloro-2-hydroxypropylsulfonic acid, sodium salt (CHPS) Epichlorohydrin (one mole, 93 g) is slowly added to an aqueous solution of one mole sodium bisulfite (104 g in about 120 ml water) while maintaining the temperature at about 80° C. The epichlorohydrin is added dropwise over about three hours, and after addition is complete, the mixture is stirred for an additional hour. After the mixture cools to about 20° C., the solids of 3-chloro-2-hydroxypropylsulfonic acid sodium salt formed from the reaction are filtered and analyzed by Nuclear Magnetic Resonance (NMR). The ISC spectra shows peaks at 69.99 ppm, 56.97 ppm, and 51.18 ppm from TMS (tetramethyl silane) and the proton spectrum shows, three groups of peaks centering at 2.95, 3.58 and 4.17 ppm (from TMS), in a 2:2:1 ratio respectively, consistent with the structure of CHPS. | |
| With sodium hydrogen sulfate In water Experimental Procedure A STEP A STEP A A mixture of 67.8 g (0.66 mol) of NaHSO3, 60.0 g of epichlorohydrin, and 135 ml of water was stirred at reflux at 7.0 hours and let cool to 22° overnight. Filtration afforded 72.3 g of sodium-2-hydroxy-3-chloro-1-propanesulfonate. | |
| With sodium hydrogensulfite In water Experimental Procedure 1 Preparation of 3-chloro-2-hydroxypropyl-sulfonic acid sodium salt EXAMPLE 1 Preparation of 3-chloro-2-hydroxypropyl-sulfonic acid sodium salt To a solution of sodium bisulfite (10 moles) in water (2500 ml) held at 80°-90° C. was added epichlorohydrin (10 moles) during 45 mins. Upon completion of the addition the mixture was heated at reflux for 1 hour. Upon cooling crystalline 3-chloro-2-hydroxypropyl-sulfonic acid sodium salt separated mp 253°-6° (decomp). | |
| With sodium sulfite In water Experimental Procedure Preparing Example 1; Sodium sulfite was dissolved in water, and epichlorohydrin was added to prepare a sodium chlorohydroxy sulfonate powder. | |
| With sodium tetrahydroborate In water at 20℃; for 1.5h; chemoselective reaction; Experimental Procedure General procedure: In a typical experiment, 0.5mmol of nitroarene and 0.002g(2mol%) NiNPs/DNA were added to 2mL water and thenstirred for 2-3min for thoroughly mixing. Subsequently,1mmol of NaBH4was added to the reaction mixture undermagnetic stirring at room temperature. The extent of thereaction was monitored by thin layer chromatography.Reproducibility of the results was checked by repeating theruns at least three times and was found to be within acceptablelimits (± 3%). When the reaction was completed, thereaction mixture was diluted with ethyl acetate and the catalystwas recovered by centrifugation. The combined organicfractions were dried over Na2SO4and evaporated underreduced pressure. The crude product was purified by columnchromatography on silica gel with a mixture of ethyl acetateand n-hexane as the eluent, and the ratio of ethyl acetate andn-hexane was depended on the structure of the products.The structure of isolated products was verified by 1H NMR. | |
| With sodium hydrogensulfite In water at 85℃; for 2h; |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H315 (91.4%): Causes skin irritation [Warning Skin corrosion/irritation] H318 (50.54%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H319 (49.46%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (91.4%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P310, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | 290559 |
| Storage | Under the room temperature and away from light |
| Shelf Life | No data available |
| Market Price | USD 21/kg |
| Use Pattern |
| 3-CHLORO-2-HYDROXYPROPANESULFONIC ACID SODIUM SALT CAS 126-83-0 often used as synthetic polymers industry in important functional monomer. |
| Used as organic chemical intermediates. |
| Used as surfactants. |
| Used as modified starch. |
| Used as drilling fluid loss materials. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |