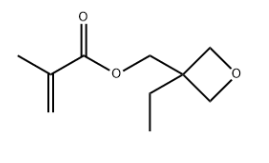
3-Ethyl-3-(methacryloyloxy)methyloxetane CAS#: 37674-57-0; ChemWhat Code: 1411590
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2012/45616 | POSITIVE PHOTOSENSITIVE RESIN COMPOSITION, METHOD FOR FORMING CURED FILM, CURED FILM, ORGANIC EL DISPLAY DEVICE AND LIQUID CRYSTAL DISPLAY DEVICE | 2012 |
Physical Data
| Appearance | Colorless clear liquid |
Spectra
No data available
Route of Synthesis (ROS)

Route of Synthesis (ROS) of 3-Ethyl-3-(methacryloyloxy)methyloxetane CAS 37674-57-0
| Conditions | Yield |
| With 2,2′-azobis-(2,4-dimethylvaleronitrile) In 3-methoxy butanol; 3-methoxybutyl acetate at 90℃; for 8h; | |
| Experimental Procedure Example 15; The inside of a 1-liter flask equipped with a reflux condenser, dropping funnels, and a stirrer was allowed to be a nitrogen atmosphere by feeding a suitable amount of nitrogen thereto; 150 parts by weight of 3-methoxy-1-butanol and 110 parts by weight of 3-methoxybutyl acetate were placed in the flask and heated to 90°C with stirring. Next, a solution was prepared by dissolving 60 parts by weight of methacrylic acid (MAA), 160 parts by weight of a 50:50 (by mole) mixture (E-DCPA) of 3,4-epoxytricyclo[5.2.1.02,6]dec-9-yl acrylate (belonging to compounds represented by Formula (1a-1)) and 3,4-epoxytricyclo[5.2.1.02,6]dec-8-yl acrylate (belonging to compounds represented by Formula (1a-2)), and 80 parts by weight of (3-ethyl-3-oxetanyl)methyl methacrylate (OXMA) in 170 parts by weight of 3-methoxybutyl acetate. The solution was added dropwise into the flask using a dropping pump over about three hours. Separately, 50 parts by weight of 2,2′-azobis(2,4-dimethylvaleronitrile) as a polymerization initiator was dissolved in 220 parts by weight of 3-methoxybutyl acetate to yield a solution, and this solution was added dropwise into the flask using another dropping pump over about five hours. After the completion of dropwise addition of the polymerization initiator, the mixture was held at the same temperature for about three hours, then cooled to room temperature, and thereby yielded a copolymer solution having a viscosity at 23°C of 98 mPa.s, a solid content of 31.5 percent by weight, and an acid value as a solution of 36.3 mg-KOH/g. The formed copolymer had a weight-average molecular weight (Mw) of 8700 and a molecular weight distribution of 2.06. |
Safety and Hazards
No data available
Other Data
| Transportation | Not dangerous goods |
| HS Code | |
| Storage | Store at room temperature, sealed and away from light. |
| Shelf Life | 2 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 184.235 |
| logP | 1.51 |
| HBA | 3 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 35.53 |
| Rotatable Bond (RotB) | 5 |
| Matching Veber Rules | 2 |
| Use Pattern |
| 3-Ethyl-3-(methacryloyloxy)methyloxetane CAS #: 37674-57-0 is a commonly used photoinitiator that can undergo copolymerization with other monomers such as methyl methacrylate and acrylic esters to form high molecular weight polymers. These polymers exhibit excellent optical properties and abrasion resistance, making them widely used in coatings, adhesives, and optical materials. UV-curable coatings prepared from 3-Ethyl-3-(methacryloyloxy)methyloxetane can rapidly cure under ultraviolet (UV) irradiation. These coatings offer good adhesion, abrasion resistance, and chemical resistance, making them suitable for coating surfaces such as wood, plastics, and metals. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |