3-Oxocyclobutanecarboxylic acid CAS#: 23761-23-1; ChemWhat Code: 22941
Identification
| Product Name | 3-Oxocyclobutanecarboxylic acid |
| IUPAC Name | 3-oxocyclobutane-1-carboxylic acid |
| Molecular Structure | 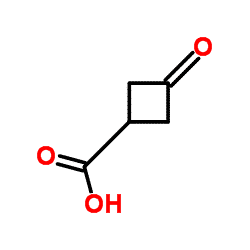 |
| CAS Registry Number | 23761-23-1 |
| EINECS Number | No data available |
| MDL Number | MFCD00100900 |
| Beilstein Registry Number | No data available |
| Synonyms | 3-oxocyclobutanecarboxylic acid3-oxocyclobutane-1-carboxylic acid3-oxocyclobutylcarboxylic acidcyclobutanone-3-carboxylic acid |
| Molecular Formula | C5H6O3 |
| Molecular Weight | 114.099 |
| InChI | InChI=1S/C5H6O3/c6-4-1-3(2-4)5(7)8/h3H,1-2H2,(H,7,8) |
| InChI Key | IENOFRJPUPTEMI-UHFFFAOYSA-N |
| Canonical SMILES | C1C(CC1=O)C(=O)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN118026938 | Pyrazolone ester compound and electrochemical synthesis method thereof | 2024 |
| WO2016/2968 | HETEROCYCLIC COMPOUNDS AND THEIR USE AS RETINOID-RELATED ORPHAN RECEPTOR (ROR) GAMMA-T INHIBITORS | 2016 |
Physical Data
| Appearance | Off-white solid |
| Solubility | It is soluble in water as well as soluble in alcohol, Slightly soluble in water. |
| Flash Point | 147°C |
| Refractive index | No data available |
| Sensitivity | No data availableive & Hygroscopic |
| Melting Point, °C | Solvent (Melting Point) |
| 69 – 70 | benzene, hexane |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz | Original Text (NMR Spectroscopy) |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | ||
| Chemical shifts | 1H | tetrachloromethane | ||
| 1H | fluorosulfonylchloride | 13C NMR (CDC13): δ 203.1; 180.1; 51.5; 27.2. | ||
| 1H | chloroform-d1 | 1H NMR (CDC13): δ 11.47 (br s, 1 H); 3.2-3.5 (m, 5 H). | ||
| Chemical shifts | 1H | CD3OD | 400 |
| Description (Mass Spectrometry) |
| gas chromatography mass spectrometry (GCMS), spectrum |
| liquid chromatography mass spectrometry (LCMS), electrospray ionisation (ESI), spectrum |
| ESI (Electrospray ionisation) |
| FAB (Fast atom bombardment), Spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In acetonitrile for 1h; Solvent; Reagent/catalyst; Inert atmosphere; | 100% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 3h; Inert atmosphere; | 97% |
| With toluene-4-sulfonic acid In toluene for 3h; Heating; | 89% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H302 (88.9%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (93.3%): Causes skin irritation [Warning Skin corrosion/irritation] H318 (84.4%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H335 (88.9%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P304+P340, P305+P354+P338, P317, P319, P321, P330, P332+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 6NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 114.101 |
| logP | -0.955 |
| HBA | 3 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 54.37 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Synthesis of antibiotics and antiviral drugs: As an intermediate containing a ring structure, it can be used to synthesize compounds with antibacterial or antiviral activity. Its ring structure can improve the biological activity and cell membrane permeability of the molecule in some cases, thereby enhancing the efficacy. |
| Synthesis of unnatural amino acids: 3-Oxocyclobutanecarboxylic acid is often used to synthesize unnatural amino acids. These special amino acids are used to develop peptide drugs, which helps to improve the stability, selectivity and metabolic half-life of the drug. |
| Anticancer drug development: Due to its unique molecular structure, 3-Oxocyclobutanecarboxylic acid is used as a key skeleton in the design of some anticancer drugs. Its ring structure can help drug molecules bind to specific protein targets, helping to inhibit the proliferation of cancer cells. |
| Synthesis of enzyme inhibitors: 3-Oxocyclobutanecarboxylic acid can be used as an intermediate for synthesizing enzyme inhibitors to help design drugs for regulating the activity of biological enzymes. Such drugs have potential in the treatment of metabolic diseases and neurodegenerative diseases such as Alzheimer’s disease. |
| Other drug molecular modifications: It can also be used to introduce cyclobutane structures into existing drug molecules to optimize pharmacokinetic properties, such as increasing bioavailability or reducing toxicity. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |