3-tert-Butylphenol CAS#: 585-34-2; ChemWhat Code: 795856
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2023/117370 | REGIOSELECTIVE SYNTHESIS OF SUBSTITUTED COMPOUNDS | 2023 |
| CN114957152 | Synthesis method of benzoxazole compound | 2022 |
| CN113443965 | Method for hydrolyzing diarylether compound to generate aryl phenol compound | 2021 |
| CN110818537 | One-pot synthetic method of phenoxyenyne ether compounds | 2020 |
| CN110981700 | Preparation method of alkylphenol compounds | 2020 |
Physical Data
| Appearance | White powder |
| Melting Point, °C | Pressure (Boiling Point), Torr |
| 125 – 130 | 20 |
| 90 – 90.5 | 2 |
| 97 – 99 | 3 |
| 129.5 | 20 |
| 98 | 3 |
| 132.5 | 20 |
| 172 | 100 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.048 | 19.85 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| UV/VIS spectrum of the complex | CH2Cl2 | 22 | ethenetetracarbonitrile |
| Stability constant of the complex with … | CH2Cl2 | 22 | ethenetetracarbonitrile |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | |
| Chemical shifts | 13C | dimethylsulfoxide-d6 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 101 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands | neat (no solvent, solid phase) | |
| Bands | neat (no solvent, solid phase) | |
| Bands | tetrachloromethane | 25 |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | dichloromethane | 400 |
| Absorption maxima |
Route of Synthesis (ROS)
| Conditions | Yield |
| With bromine In dichloromethane for 1h; | 100% |
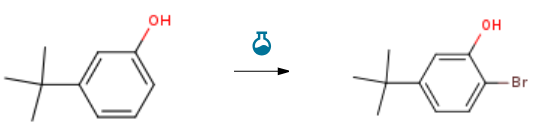
| With bromine In tetrachloromethane at 5℃; for 1h; Experimental Procedure To a cooled solution of 3-tert-butylphenol (5 g, 33.3 mmol) in carbon tetrachloride (CARE. 40 ml) at “50C was added bromine (CARE. 1.7 ml, 33.3 mmol) portion-wise over 30 minutes and the reaction was allowed to stir at “5° C for a further 30 minutes. The reaction was partioned between water and dichioromethane and the organic layer was washed with water until pH neutral before being dried (MgSO4) and concentrated in vacuo to give the title compound as a yellow liquid (7 g, 92 %). 1H NMR (DMSO) δ 7.35-7.37 (1 H1 m), 7.05-7.06 (1 H, m), 6.83-6.86 (1 H, m), 1.29 (9 H, s); m/z 227.24/229.22 (MHO | 92% |
| With bromine In dichloromethane at 0℃; Inert atmosphere; | 91% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H314 (100%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] H318 (43.18%): Causes serious eye damage [Danger Serious eye damage/eye irritation] |
| Precautionary Statement Codes | P260, P264, P264+P265, P280, P301+P330+P331, P302+P361+P354, P304+P340, P305+P354+P338, P316, P317, P321, P363, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under room temperature away from light |
| HS Code | |
| Storage | Under room temperature away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 150.221 |
| logP | 3.433 |
| HBA | 0 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 20.23 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Use Pattern |
| 3-tert-Butylphenol CAS#: 585-34-2 can serve as an antioxidant, playing a role in preventing oxidation reactions. Antioxidants help protect substances from the effects of oxygen or other oxidizing agents, preventing oxidative damage. It may be used as an inhibitor in polymerization reactions, particularly in some polymer production processes. It can slow down or inhibit free radical polymerization reactions, aiding in controlling the molecular weight and other properties of polymers. Due to its antioxidant properties, 3-tert-Butylphenol might be used in rubber and rubber products to extend their lifespan and prevent aging and degradation. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |