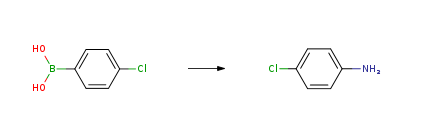
4-Chloroaniline CAS#: 106-47-8; ChemWhat Code: 78943
Identification
| Product Name | 4-Chloroaniline |
| IUPAC Name | 4-chloroaniline |
| Molecular Structure | 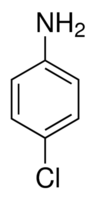 |
| CAS Registry Number | 106-47-8 |
| EINECS Number | No data available |
| MDL Number | MFCD00007835 |
| Beilstein Registry Number | No data available |
| Synonyms | 4-chloro-aniline, 4-chloroaniline, p-Chloroaniline |
| Molecular Formula | ClC6H4NH2 |
| Molecular Weight | 127.57 |
| InChI | InChI=1S/C6H6ClN/c7-5-1-3-6(8)4-2-5/h1-4H,8H2 |
| InChI Key | QSNSCYSYFYORTR-UHFFFAOYSA-N |
| Canonical SMILES | c1cc(ccc1N)Cl |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN112321436 | Method for synthesizing heteroatom-substituted aromatic compounds from styrene compounds | 2021 |
| CN110683969 | Urea derivative and synthesis method thereof (by machine translation) | 2020 |
| CN110804035 | Preparation method and application of tetrahydrobenzofuran Mannich base compound. (by machine translation) | 2020 |
Physical Data
| Appearance | White or milky white flake crystals |
| Solubility | 0.3 g/100 mL (20 ºC) |
| Flash Point | 120 ºC |
| Refractive index | 1.5546 |
| Melting Point, °C | Solvent (Melting Point) |
| 73 – 75 | |
| 69 – 72 | |
| 71 | |
| 65 – 67 | |
| 70 | Petroleum ether |
| 68 – 69 | ethyl acetate, hexane |
| 69 – 71 | methanol |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 232 | |
| 70 – 72 | |
| 98 – 100 | 10 |
| 102 | 10 |
| 120 | 19 |
| 232.3 | 760 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.21453 | ||
| 1.48 | ||
| 1.427 | 4 | 20 |
| 1.22 | 4 | 25 |
| 1.36 | 4 | 20 |
| 1.175 | 4 | 70 |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C |
| 1.5512 | 656.3 | 100 |
| 1.5576 | 587.6 | 100 |
| 1.5744 | 486.1 | 100 |
| 1.5546 | 656.3 | 87.2 |
| 1.5779 | 486.1 | 87.2 |
| 1.5939 | 434 | 87.2 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Formation constant of a complex | CHCl3 | 25 | 2C15H13Br2N4O(1-)*Ni(2+) |
| IR spectrum of the complex | CCl4 | 24.8 | N,N-dimethyl-formamide |
| Stability constant of the complex with … | CCl4 | 24.85 | N,N-dimethyl-formamide |
| Stability constant of the complex with … | CCl4 | 24.5 | dimethyl sulfoxide |
| IR spectrum of the complex | CCl4 | 24.85 | N,N,N,N,N,N-hexamethylphosphoric triamide |
| IR spectrum of the complex | solid | 26.9 | naphthalene |
| Stability constant of the complex with … | nitromethane | 24.9 | 2,4,6-Trinitrophenol |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 |
| Chemical shifts, Spectrum | 13C | dimethylsulfoxide-d6 | 125 |
| Spectrum | 1H | water-d2 | |
| Chemical shifts | 13C | chloroform-d1 | 100 |
| Chemical shifts, Spectrum | 1H | dichloromethane-d2 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | neat (no solvent, solid phase) | |
| Spectrum | potassium bromide | |
| ATR (attenuated total reflectance), Bands | ||
| Mid IR (MIR), Bands | potassium bromide | |
| Bands | KBr | 3470 – 3380 1/cm |
| Bands | CCl4 | 3486 – 1619 cm**(-1) |
| Spectrum | CCl4 | 3600 – 2000 cm**(-1), IR-Spektrum der deuterierten Verbindung. |
| Description (Mass Spectrometry) |
| high resolution mass spectrometry (HRMS), electrospray ionisation (ESI), time-of-flight mass spectra (TOFMS), spectrum |
| gas chromatography mass spectrometry (GCMS), electron impact (EI), spectrum |
| electrospray ionisation (ESI), spectrum |
| gas chromatography mass spectrometry (GCMS), spectrum |
| electrospray ionisation (ESI), spectrum |
| CID (collision-induced dissociation), electrospray ionisation (ESI), liquid chromatography mass spectrometry (LCMS), spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | water | |
| Spectrum | H2O | |
| Absorption maxima | methylcyclohexane | 318.1 |
| Absorption maxima | methylcyclohexane, dioxane | 315.1 |
| Absorption maxima | dioxane, methylcyclohexane | 300.09, 244.08 |
Route of Synthesis (ROS)
| Conditions | Yield |
| With potassium carbonate; ammonium hydroxide In methanol at 60℃; for 21h; | 92% |
| With copper(ll) sulfate pentahydrate; ammonia; sodium hydroxide In water at 20℃; under 760.051 Torr; for 5h; | 90% |
| With ammonium hydroxide; potassium nitrate In water at 20℃; for 2h; Electrochemical reaction; chemoselective reaction; | 80% |
| With sodium hydroxide; hydroxylamine-O-sulfonic acid In water; acetonitrile at 100℃; for 0.25h; Microwave irradiation; | 74% |
| With copper(I) oxide; ammonium hydroxide In water for 0.0833333h; Microwave irradiation; |
Safety and Hazards
| Pictogram(s) |     |
| Signal | Danger |
| GHS Hazard Statements | H301: Toxic if swallowed [Danger Acute toxicity, oral] H311: Toxic in contact with skin [Danger Acute toxicity, dermal] H317: May cause an allergic skin reaction [Warning Sensitization, Skin] H331: Toxic if inhaled [Danger Acute toxicity, inhalation] H350: May cause cancer [Danger Carcinogenicity] H400: Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410: Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P201, P202, P261, P264, P270, P271, P272, P273, P280, P281, P301+P310, P302+P352, P304+P340, P308+P313, P311, P312, P321, P322, P330, P333+P313, P361, P363, P391, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 6.1; Packaging Group: II; UN Number: 2018 |
| Under the room temperature and away from light | |
| HS Code | 292142 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Use Pattern |
| 4-Chloroaniline CAS#: 106-47-8 toxic |
| 4-Chloroaniline CAS#: 106-47-8 carcinogenic |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watsonnoke Scientific Ltd | https://www.watsonnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |