4-Fluoro-3-nitroaniline CAS#: 364-76-1; ChemWhat Code: 23573
Identification
| Product Name | 4-Fluoro-3-nitroaniline |
| IUPAC Name | 4-fluoro-3-nitroaniline |
| Molecular Structure | |
| CAS Registry Number | 364-76-1 |
| EINECS Number | 206-665-5 |
| MDL Number | MFCD00007833 |
| Beilstein Registry Number | 2210199 |
| Synonyms | 4-Fluoro-3-nitroaniline 364-76-1 3-Nitro-4-fluoroaniline 4-Fluoro-3-nitrobenzenamine Benzenamine, 4-fluoro-3-nitro- Aniline, 4-fluoro-3-nitro- 4-Fluoro-3-nitro-aniline 4-fluoro-3-nitrophenylamine 4-Fluoro-3-nitro-phenylamine MFCD00007833 R8DY9W4LRV DTXSID5052042 NSC-10293 4-Fluoro-3-nitro-benzamine; 3-Nitro-4-fluoroaniline 5-amino-2-fluoronitrobenzene 3-nitro-4-fluoroanailine EINECS 206-665-5 NSC 10293 3-nitro-4-fluoro aniline 4-fluoro-3-nitro aniline BRN 2210199 4-Amino-2-nitrofluorobenzene 4-fluoro-5-nitroaniline UNII-R8DY9W4LRV 4-fluor-3-nitro-aniline 4-fluoro- 3-nitroaniline 4-12-00-01668 (Beilstein Handbook Reference) SCHEMBL394329 (4-fluoro-3-nitrophenyl)amine DTXCID6030609 4-Fluoro-3-nitroaniline, 97% CHEBI:48642 4-F-3NA BCP18823 NSC10293 Tox21_303736 STL352282 AKOS000119363 AC-3742 CS-W019572 NCGC00357045-01 AS-11938 CAS-364-76-1 DB-028561 AM20060291 F0168 NS00041701 EN300-17970 A19812 D77856 AN-584/43460752 W-106609 Q27121300 Z57127374 F2190-0476 InChI=1/C6H5FN2O2/c7-5-2-1-4(8)3-6(5)9(10)11/h1-3H,8H |
| Molecular Formula | C6H5FN2O2 |
| Molecular Weight | 156.11 |
| InChI | InChI=1S/C6H5FN2O2/c7-5-2-1-4(8)3-6(5)9(10)11/h1-3H,8H2 |
| InChI Key | LLIOADBCFIXIEU-UHFFFAOYSA-N |
| Isomeric SMILES | C1=CC(=C(C=C1N)[N+](=O)[O-])F |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2014/57911 | NOVEL 4,6-DISUBSTITUTED AMINOPYRIMIDINE DERIVATIVES HAVING BOTH AROMATIC AND HALOGENIC SUBSTITUENTS | 2014 |
| US2011/86853 | Therapeutic Compounds | 2011 |
| US2007/249868 | New process for the preparation of 4-hydroxalkylamino-2-nitro-anisoles | 2007 |
| US2007/244092 | Therapeutic Compounds | 2007 |
| WO2005/97051 | SULFIDE DYES | 2005 |
Physical Data
| Appearance | Yellow crystals powder |
| Melting Point, °C | Solvent (Melting Point) |
| 142 – 145 | |
| 200 – 205 | |
| 96.5 – 97.5 | |
| 98 | |
| 97 – 98.2 | H2O |
| 96 |
| Boiling Point, °C |
| 10 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 100 |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 400 |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 300 |
| 1H | dimethylsulfoxide-d6 | 300 |
| Description (IR Spectroscopy) |
| Bands |
| Spectrum |
| Description (UV/VIS Spectroscopy) |
| Absorption maxima |
Route of Synthesis (ROS)
| Conditions | Yield |
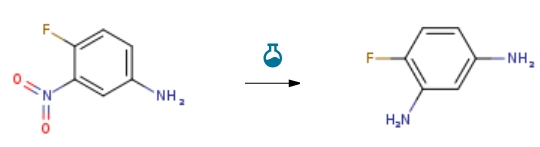
| With iron(III) chloride hexahydrate; pyrographite; hydrazine hydrate In ethanol at 70℃; Experimental Procedure 31.1 Example 31 1. Weigh 1.6 g (10 mmol) of 4-fluoro-3-nitroaniline, 1.5 g of activated carbon and 0.1 g of FeCl3·6H2O into a round-bottomed flask, add 25 mL of absolute ethanol, heat up to 70°C, and then slowly 2.9 mL of 80% hydrazine monohydrate was added dropwise to the reaction system, and the reaction was carried out at 70° C. for 1 h.The filtrate was collected by suction filtration, dried and concentrated to obtain 1.2 g of 4-fluoro-3-aminoaniline as a yellow-green solid with a yield of 95.0%. | 95% |
| With silver tetrafluoroborate; 4,4′-Dimethoxy-2,2′-bipyridin; potassium tert-butylate; hydrogen In 1,4-dioxane at 80℃; under 30003 Torr; for 48h; Autoclave; Experimental Procedure 15 Example 15 In Example 1, the m-nitroacetophenone used was replaced with equimolar 4-fluoro-3-nitroaniline,the reaction was carried out at 80 °C for 48 hours at 4.0 MPa. The other procedure was the same as in Example 1 to give 4-fluoro-1,3-diaminobenzene in a yield of 94%, _: A solution of 16.44 mg (0.04 mmol) 4,4′-dimethoxy-2,2′-bipyridine silver 11.22 mg (0.1 mmol) of potassuim t-butoxide and 1 mL of 1,4-dioxane were charged into an autoclave. After stirring, 165.15 mg (1 mmol) of m-nitroacetophenone was added and the mixture was stirred at 80 °C. The reaction was carried out for 8 hours. After the reaction has finishedm the reaction solution was extracted with water and dichloromethane to collect the organic phase. Then, the organic phase was dried over anhydrous Na2SO4, suction filtered, rotary evaporated and chromatographed to give a yellow solid 3-acetanilide. Yield 96 %. | 94% |
| With sodium hydroxide In hydrogenchloride Experimental Procedure 2 Preparation of 4-fluoro-m-phenylenediamine EXAMPLE 2 Preparation of 4-fluoro-m-phenylenediamine To a solution of 9 grams of SnCl2.2H2 O in 12 ml of concentrated HCl, are added in a single batch, with stirring, 1.6 grams of 4-fluoro-3-nitroaniline, which dissolves as a brisk reaction occurs, the temperature reaching 95° to 100° C. The reaction mixture is allowed to cool to room temperature, and poured into 70 ml of 50% NaOH solution in ice, so that the temperature remains below 20° C. The resultant strongly alkaline solution is extracted 3 times with 50 ml of ethyl ether. The ethyl ether extracts are combined, washed with 30 ml of distilled water and dried with anhydrous sodium sulfate. The ethyl ether solution is evaporated to dryness and 1.2 gram of a dark colored oil is obtained. | |
| With sodium tetrahydroborate In water Darkness; Experimental Procedure 2.4. Reduction of organic pollutants in aqueous solution General procedure: The catalytic activity of the prepared catalyst was evaluated towardsthe reduction of 4-nitrophenol (4-NP) as a model system in an aqueoussolution. All the experiments were performed in dark conditions using astandard 3 mL quartz cuvette with a 1 cm path length which was placedin the dark compartment of the spectrophotometer. In a typical run, 1mg of the catalyst MS 3 Å/Cu0 was dispersed in an aqueous solution of25 mL of 0.2 mM of the nitro compound by mechanical effect of ultrasonicwaves using a sonicator, for one minute. Different concentrationsof freshly prepared NaBH4 with a volume equal to 25 mL were addedindividually to the initial solution. 4 mL of this reaction mixture wasimmediately transferred in a quartz cuvette and its absorbance spectrawere recorded using a UV-vis spectrophotometer (UV-vis Specord 210Analytik Jena). The UV-vis absorbance spectra were recorded in the200-700 nm range after regular time intervals at room temperature. To show more the effectiveness of the catalyst, the optimized reactionconditions were applied in the reduction of 2-nitrophenol (2-NP), 2-nitroaniline (2-NA), 4-fluoro-2-nitroaniline (4-F-2-NA), 4-fluoro-3-nitroaniline (4-F-3-NA) and Methylene Blue dye (MB). | |
| With hydrogen In ethanol at 20℃; under 750.075 Torr; | > 99 %Spectr |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302 (95.83%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (18.75%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (18.75%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (18.75%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Store at room temperature for long time, in container tightly sealed; Protect from light. |
| HS Code | |
| Storage | Store at room temperature for long time, in container tightly sealed; Protect from light. |
| Shelf Life | 2 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 156.116 |
| logP | 1.258 |
| HBA | 1 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 71.84 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Use Pattern |
| 4-Fluoro-3-nitroaniline is commonly used as an intermediate in the synthesis of pharmaceuticals. It serves as a key raw material in the production of antibacterial, antitumor, and other therapeutic drugs. 4-Fluoro-3-nitroaniline is used in the synthesis of agrochemicals, serving as a precursor for insecticides, herbicides, and fungicides. Modifying its structure can lead to the development of highly efficient and low-toxicity agricultural products. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |