4-(Methylthio)phenol CAS#: 1073-72-9; ChemWhat Code: 78933
Identification
| Product Name | 4-(Methylthio)phenol |
| IUPAC Name | 4-methylsulfanylphenol |
| Molecular Structure | 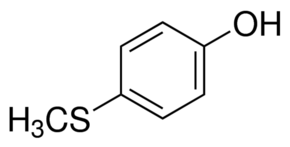 |
| CAS Registry Number | 1073-72-9 |
| EINECS Number | 214-031-4 |
| MDL Number | MFCD00002351 |
| Beilstein Registry Number | 2041507 |
| Synonyms | 4-Hydroxythioanisole;p-(Methylthio)phenol;Phenol, 4-(methylthio)- [ACD/Index Name];Phenol, p-(methylthio)- p-Hydroxythioanisole;QR DS1 [WLN];SM1578500;3-ACETAMIDE THIOANISOLE |
| Molecular Formula | C7H8OS |
| Molecular Weight | 140.203 |
| InChI | InChI=1S/C7H8OS/c1-9-7-4-2-6(8)3-5-7/h2-5,8H,1H3 |
| InChI Key | QASBCTGZKABPKX-UHFFFAOYSA-N |
| Canonical SMILES | CSc1ccc(cc1)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN109438190 | A Lewis acid-catalyzed phenol derivative activation method and application (by machine translation) | 2019 |
| WO2018/170067 | SMALL MOLECULE SENSITIZATION OF BAX ACTIVATION FOR INDUCTION OF CELL DEATH | 2018 |
| CN106905202 | A synthetic method of the compound sulfones (by machine translation) | 2017 |
| US2005/54707 | Pyrazole derivatives | 2005 |
| US2003/40639 | Method for the preparation of substituted benzene derivatives | 2003 |
Physical Data
| Appearance | White or off-white crystalline powder |
| Solubility | Soluble in water 9.59 g/ l. |
| Flash Point | 153-156°C/20mm |
| Refractive index | 1.5530 (estimate) |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) |
| 85 | |
| 85 – 86 | |
| 76 – 79 | |
| 86 – 87 | benzene, toluene |
| 84 – 85 | heptane |
| 83 – 84 | petroleum ether |
| 84 – 85 | petroleum ether |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 152.5 – 154 | 19 |
| 115 – 116 | 3 |
| 135 – 140 | 16 |
| 146 – 148 | 10 |
| 113 | 6 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.21 | 25 |
| 1.3 | 20 |
| Chromatographic data | Original string |
| TLC (Thin layer chromatography) | Rf=0.35 (Hexanes:EtOAc=4:1); |
| Dissociation Exponent (pK) | Temperature (Dissociation Exponent), °C | Solvent (Dissociation Exponent) | Method (Dissociation Exponent) | Type (Dissociation Exponent) | Comment (Dissociation Exponent) |
| 9.61 | 25 | spectrophotometric a | a1/apparent | ||
| (pk’)pKa | |||||
| (k’)scheinbare Diss.-Konst. in 48percentig. A. fuer den Grundzustand, den 1. angeregten Singulettzustand u. den niedrigsten Triplettzustand (S. 43) | |||||
| 9.47 | 25 | H2O | potentiometric | a/apparent | |
| 9.53 | 25 | H2O | potentiometric | a/apparent |
| Description (Electrical Moment) | Moment (Electrical Moment), D | Temperature (Electrical Moment), °C | Solvent (Electrical Moment) |
| Dipole moment | 2.1 | 40 | benzene |
| Solvent (Association (MCS)) | Temperature (Association (MCS)), °C |
| aq. ethanol | 25 |
Spectra
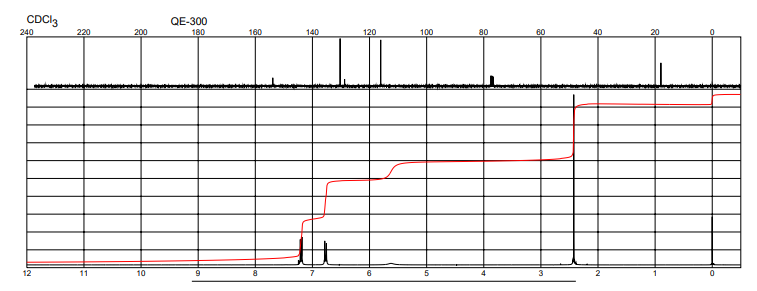
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 100 |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 500 |
| Chemical shifts | 13C | dimethylsulfoxide-d6 | 125 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 101 |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | 400 |
| Chemical shifts, Spectrum | 13C | dimethylsulfoxide-d6 | 125 |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 13C | chloroform-d1 | 100 |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 300 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 75 |
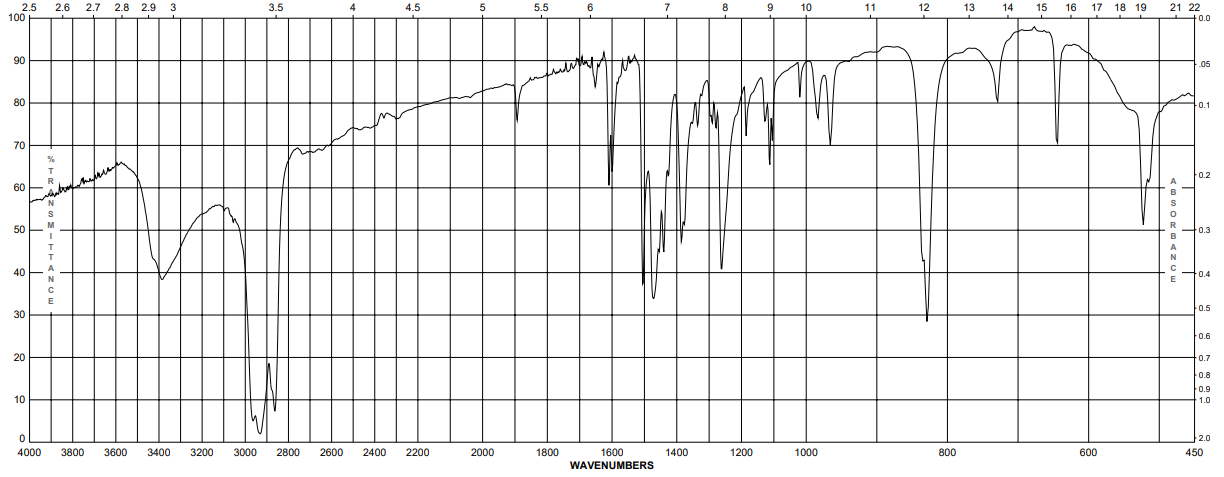
| Description (IR Spectroscopy) |
| IR |
| Description (Mass Spectrometry) |
| gas chromatography mass spectrometry (GCMS), electron impact (EI), spectrum |
| electron impact (EI), spectrum |
| high resolution mass spectrometry (HRMS), electron impact (EI), time-of-flight mass spectra (TOFMS), spectrum |
| ESI (Electrospray ionisation), LCMS (Liquid chromatography mass spectrometry), TOFMS (Time of flight mass spectrum), Spectrum |
| APCI (atmospheric pressure chemical ionization), LCMS (Liquid chromatography mass spectrometry), TOFMS (Time of flight mass spectrum), Spectrum |
| GCMS (Gas chromatography mass spectrometry), EI (Electron impact), Spectrum |
| chemical ionization (CI) |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Absorption maxima | aq. ethanol | 230, 255, 285 | 6920, 7080, 1070 |
| Absorption maxima | ethanol | 255.2 | |
| Absorption maxima | aq. NaOH | 259.5 | |
| Absorption maxima | sodium ethanolate / ethanol | 262.7 | |
| Absorption maxima | cyclohexane | ||
| Spectrum | ethanol |
Route of Synthesis (ROS)
| Conditions | Yield |
| With 2,5-dimethylfuran; zinc(II) phthalocyanine; oxygen; citric acid In tetrahydrofuran at 25℃; under 760.051 Torr; for 1.5h; Reagent/catalyst; Irradiation; Sealed tube; Schlenk technique; | 95% |
| With urea hydrogen peroxide adduct In acetonitrile at 27 – 29℃; for 0.0833333h; Green chemistry; chemoselective reaction; Experimental Procedure 2.1 Solution phase protocol: General procedure: To a stirred solution of aryl/alkyl boronic acid (1 mmol) in methanol or acetonitrile solvent (1 ml) was added 1 equiv. of Urea-Hydrogen peroxide (UHP) (2.5 equiv for alkyl boronic acids) at room temperature and the progress of the reaction was monitored by TLC. After completion, the reaction mixture was diluted with water and extracted with dichloromethane (DCM). The combined organic layer was dried over anhydrous sodium sulfate (Na2SO4), evaporated and subjected to silica gel column chromatography for further purification. | 92% |
| With bis-triphenylphosphine-palladium(II) chloride; potassium fluoride; carbon monoxide; oxygen In water; tert-butyl alcohol at 40℃; under 760.051 Torr; for 12h; Schlenk technique; | 91% |
| With menadione; sodium hydrogencarbonate; sodium L-ascorbate In ethanol; water at 20℃; under 760.051 Torr; for 24h; pH=8.5; Darkness; Green chemistry; | 89% |
| With N,N-dimethyl-p-toluidine N-oxide In dichloromethane at 20℃; for 0.0166667h; | 86% |
| With water; caesium carbonate; hydrazine hydrate at 80℃; for 36h; | 85% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (96.55%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 6.1; Packaging Group: II; UN Number: 3335 |
| Under the room temperature and away from light | |
| HS Code | 293090 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Use Pattern |
| 4-(Methylthio)phenol CAS#: 1073-72-9 is as an intermediate in organic synthesis |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watsonnoke Scientific Ltd | https://www.watsonnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |