4,4′-Dihydroxybenzophenone CAS#: 611-99-4; ChemWhat Code: 62704
Identification
| Product Name | 4,4′-Dihydroxybenzophenone |
| IUPAC Name | bis(4-hydroxyphenyl)methanone |
| Molecular Structure | 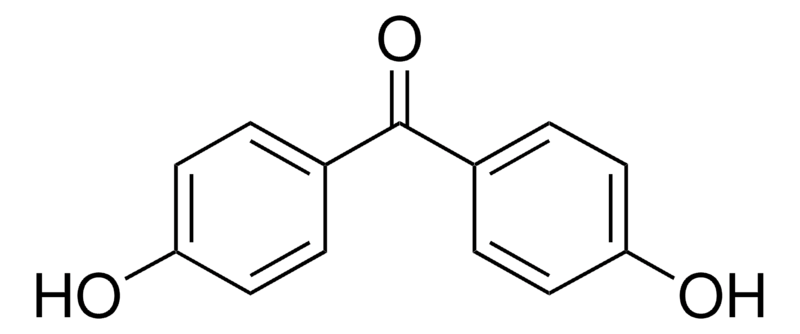 |
| CAS Registry Number | 611-99-4 |
| EINECS Number | 210-288-1 |
| MDL Number | MFCD00002358 |
| Beilstein Registry Number | No data available |
| Synonyms | 3-Pyridinamin;3-Pyridinamine;3-Pyridinamine;pyridin-3-amine;T6NJ CZ;3- Aminopyridine;3-Amino-pyridine;3-pyridylamine;Amino-3 pyridine;m-Aminopyridine;MS/MS-1064463;Pyridin-3-ylamine;Pyridine, 3-amino-;β-Aminopyridine 462-08-8 |
| Molecular Formula | C13H10O3 |
| Molecular Weight | 214.220 |
| InChI | InChI=1S/C13H10O3/c14-11-5-1-9(2-6-11)13(16)10-3-7-12(15)8-4-10/h1-8,14-15H |
| InChI Key | RXNYJUSEXLAVNQ-UHFFFAOYSA-N |
| Canonical SMILES | O=C(c1ccc(O)cc1)c1ccc(O)cc1 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| EP1248313 | Polymer electrolyte and process for producing the same | 2002 |
| US4757150 | Polyetherimide bisphenol compositions | 1988 |
Physical Data
| Appearance | White crystalline powder |
| Solubility | insoluble |
| Refractive index | 1.5090 (estimate) |
| Sensitivity | No data available |
| Melting Point, °C |
| 219 |
| 214 – 217 |
| 213 – 215 |
| Boiling Point, °C |
| 251 |
| 250 – 252 |
| Density, g·cm-3 | Type (Density) |
| 1.336 | crystallographic |
| Description (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Further physical properties of the complex | 23 | anti-fluorescyl monoclonal antibody 66D2 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | |
| Chemical shifts, Spectrum | 13C | dimethylsulfoxide-d6 | |
| Chemical shifts | 1H | [D3]acetonitrile | |
| Chemical shifts | 13C | [D3]acetonitrile |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | neat (no solvent, solid phase) | |
| Mid IR (MIR), Bands | chloroform | |
| Bands | CCl4 | 1370 – 1230 cm**(-1) |
| Description (Mass Spectrometry) |
| liquid chromatography mass spectrometry (LCMS), spectrum |
| gas chromatography mass spectrometry (GCMS), liquid chromatography mass spectrometry (LCMS), spectrum |
| liquid chromatography mass spectrometry (LCMS), time-of-flight mass spectra (TOFMS), spectrum |
| high resolution mass spectrometry (HRMS), electron impact (EI), spectrum |
| high resolution mass spectrometry (HRMS), electrospray ionisation (ESI), spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | 382 | ||
| Spectrum | film | ||
| Spectrum | methanol | 297 | |
| Spectrum | acetonitrile | 344, 560 | |
| film | 275 |
Route of Synthesis (ROS)
| Conditions | Yield |
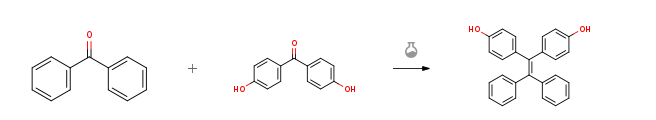
| With titanium tetrachloride; zinc In tetrahydrofuran at -78 – 70℃; for 23h; McMurry Reaction; Inert atmosphere; | 85% |
| With titanium tetrachloride; zinc In tetrahydrofuran at 20℃; Inert atmosphere; Cooling with ice; | 74% |
| With pyridine; titanium tetrachloride; zinc In tetrahydrofuran McMurry cross coupling reaction; Heating; | 69% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H300: Fatal if swallowed [Danger Acute toxicity, oral] H301: Toxic if swallowed [Danger Acute toxicity, oral] H311: Toxic in contact with skin [Danger Acute H315 (84.1%): Causes skin irritation [Warning Skin corrosion/irritation] H317 (68.3%): May cause an allergic skin reaction [Warning Sensitization, Skin] H319 (98.4%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (82.5%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] H412 (12.7%): Harmful to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P264+P265, P271, P272, P273, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P333+P317, P337+P317, P362+P364, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 214.221 |
| logP | 2.764 |
| HBA | 1 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 57.53 |
| Rotatable Bond (RotB) | 2 |
| Matching Veber Rules | 2 |
| Use Pattern |
| UV absorber This compound is often used as a UV absorber, especially in sunscreen products and polymer materials to prevent UV-induced degradation. In plastics, rubbers and coatings, 4,4′-dihydroxybenzophenone can be used as an additive to enhance the anti-aging and light stability of the material and extend its service life. |
| Pharmaceutical intermediates 4,4′-Dihydroxybenzophenone can be used as a pharmaceutical intermediate for the synthesis of antibacterial, anti-inflammatory and antioxidant drugs. The presence of its hydroxyl and carbonyl groups makes it a key starting material for the preparation of biologically active molecules (such as benzimidazole, quinazoline, etc.). |
| Building blocks in organic synthesis As an important building block in organic synthesis, this compound can be used to prepare various organic ligands and functional materials. Since the phenolic hydroxyl group in its structure can participate in a variety of chemical reactions, such as acylation, alkylation and condensation reactions, it has important applications in the synthesis of heterocyclic compounds and dyes. |
| Polymer Stabilizer In the polymer industry, 4,4′-dihydroxybenzophenone is used as an antioxidant and light stabilizer, especially for improving the light resistance and anti-aging properties of materials such as polyurethane, polyester and polystyrene. This additive can effectively prevent the degradation of materials under ultraviolet radiation and is particularly suitable for plastics and coatings for outdoor applications. |
| Functional dyes and pigments The compound can also be used to synthesize dyes and pigments with photoluminescent properties for use in optical materials and electronic displays. The structure of its phenolic hydroxyl group can be further modified to optimize the color properties and stability of the dye. |
| Analytical reagents In analytical chemistry, it can be used as a colorimetric reagent or standard to detect the content of certain metal ions or organic matter. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |