4,5-Bis(diphenylphosphino)-9,9-dimethylxanthene CAS#: 161265-03-8; ChemWhat Code: 38678
Identification
| Product Name | 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene |
| IUPAC Name | (5-diphenylphosphanyl-9,9-dimethylxanthen-4-yl)-diphenylphosphane |
| Molecular Structure | 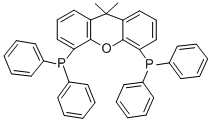 |
| CAS Registry Number | 161265-03-8 |
| Synonyms | XANTPHOS, 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene, [5-(diphenylphosphanyl)-9,9-dimethyl-9H-xanthen-4-yl]diphenylphosphane, (5-diphenylphosphanyl-9,9-dimethyl-xanthen-4-yl)-diphenyl-phosphane, (5-diphenylphosphanyl-9,9-dimethyl-xanthen-4-yl)-diphenylphosphane, 1,1′-(9,9-dimethyl-9H-xanthene-4,5-diyl)bis(1,1-diphenylphosphine), 1,1′-(9,9-dimethyl-9H-xanthene-4,5-diyl)bis[1,1-diphenylphosphine], 1,1′-(9,9-dimethyl-9H-xanthene-4,5-diyl)bis[1,1]-diphenylphosphine |
| Molecular Formula | C39H32OP2 |
| Molecular Weight | 578.63 |
| InChI | InChI=1S/C39H32OP2/c1-39(2)33-25-15-27-35(41(29-17-7-3-8-18-29)30-19-9-4-10-20-30)37(33)40-38-34(39)26-16-28-36(38)42(31-21-11-5-12-22-31)32-23-13-6-14-24-32/h3-28H,1-2H3 |
| InChI Key | CXNIUSPIQKWYAI-UHFFFAOYSA-N |
| Canonical SMILES | CC1(C2=C(C(=CC=C2)P(C3=CC=CC=C3)C4=CC=CC=C4)OC5=C1C=CC=C5P(C6=CC=CC=C6)C7=CC=CC=C7)C |
| Investigated characteristic(s) | Classification of catalysis | Type of reaction (Catalyst Investigation) |
| Turnover frequency | Homogeneous catalysis | Hydroformylation |
| Catalytic activity | Heck reaction |
Physical Data
| Appearance | Off-white or light yellow powder |
| Boiling Point | 665.7 °C at 760 mmHg |
| Flash Point | 450°C |
| Solubility | Soluble in organic solvents |
| Reaction Suitability | Reagent type: ligand Reaction type: Buchwald-Hartwig Cross Coupling Reaction Reagent type: ligand Reaction type: C-X Bond Formation Reagent type: ligand Reaction type: Hydroformylations Reagent type: ligand Reaction type: Miyaura Borylation Reaction Reagent type: ligand Reaction type: Stille Coupling Reagent type: ligand Reaction type: Suzuki-Miyaura Coupling |
| Melting Point, °C | Solvent (Melting Point) |
| 229 – 230 | |
| 230 – 232 | CHCl3, propan-2-ol |
| Density, g·cm-3 | Type (Density) |
| 1.21 | crystallographic |
| Description (Crystal Phase) | Comment (Crystal Phase) |
| Crystal structure determination | a=18.94 Angstroem, b=19.16 Angstroem, c=8.77 Angstroem, n=4., Temperature: 293 K. Method of determination: Single Crystal X-ray Diffraction |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C |
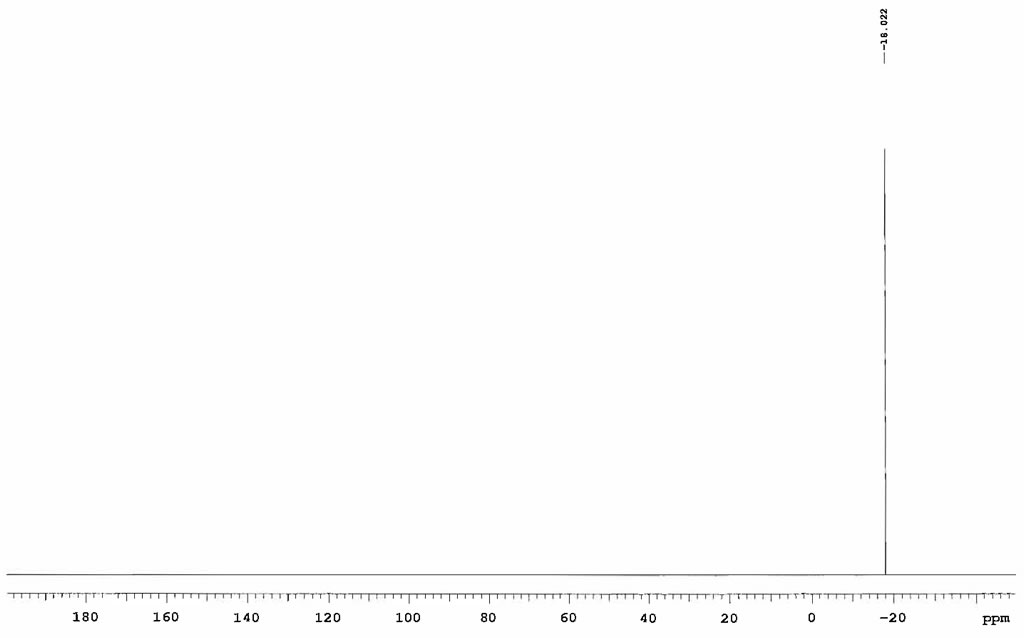
| Chemical shifts, Spectrum | 31P | dichloromethane-d2 | 23.84 |
| Chemical shifts | 31P | CD2Cl2 | 25 |
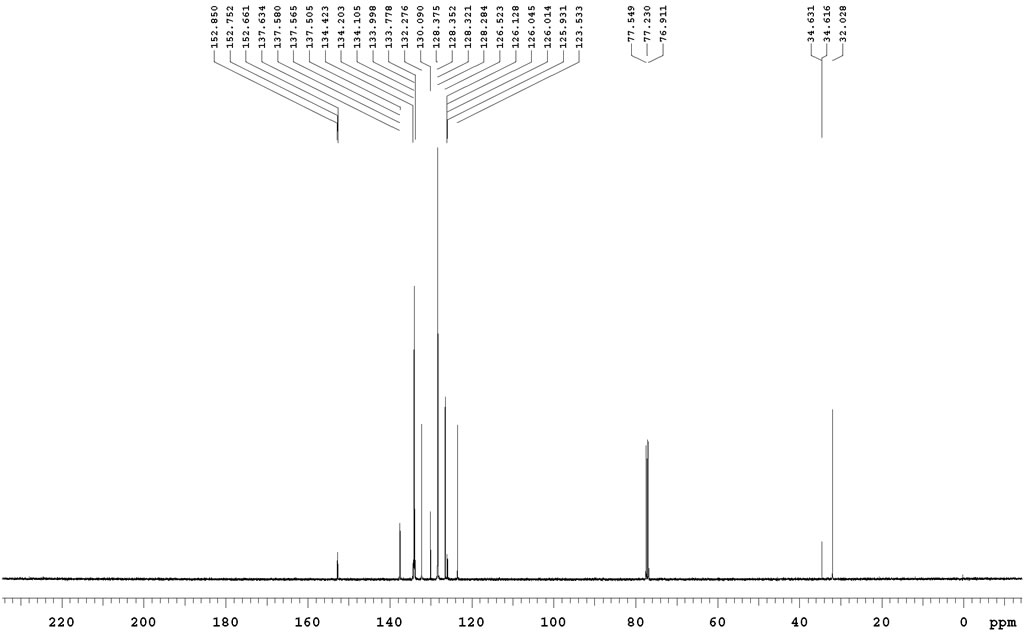
| Chemical shifts | 13C | CD2Cl2 | 25 |
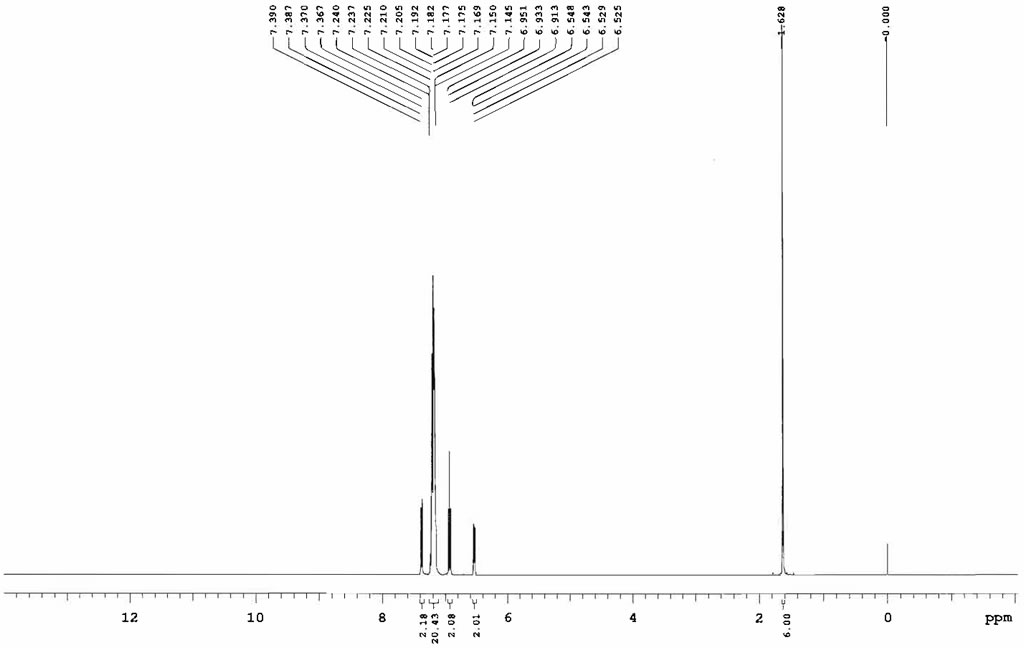
| Chemical shifts | 1H | CD2Cl2 | 25 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | potassium bromide |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | N,N-dimethyl-formamide | |||
| Spectrum | 1,2-dichloro-ethane | |||
| CH2Cl2 | ambient temperature | 262 | 23667 |
Route of Synthesis (ROS)
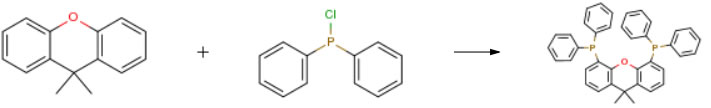
| Conditions | Yield |
| Stage #1: 9,9-dimethylxanthene With N,N,N,N,-tetramethylethylenediamine; sec.-butyllithium In tert-butyl methyl ether; cyclohexane at 6 – 10℃ Stage #2: In cyclohexane; water at 20℃ for 14h Stage #3: chloro-diphenylphosphine In cyclohexane; water at 10 – 20℃ for 5.16667h Experimental Procedure Preparation of 9,9-dimethyl-4,5-bis(diphenylphosphino)xanthene, Xantphos To a 5L round bottom flask (RBF) are added MTBE (2.5 L), 9,9-dimethylxanthene (131.4 g, 0.60 mole) and TMEDA (155 g, 1.32 mole). After degassing the solution, s-BuLi (1.11 L, 1.3 M in cyclohexane, 1.44 mole) is cannulated into a dropping funnel and then slowly added over 60 min while maintaining the batch temperature at 6-10° C. The mixture is then aged for 14 h at room temperature. Ph2PCl is added slowly via a dropping funnel while maintain the mildly exothermic reaction at 10-20° C. ~60percent of the Ph2PCl (175 mL, 0.93 mole) is added in 0.5 hour. The mixture is aged for 10 minutes before addition of the remaining Ph2PCl (120 mL, 0.63 mole). After aged for 5 h at room temperature, the reaction is quenched with MeOH (9.9 mL, 0.24 mole). The product is filtered and the slightly yellow solid is washed consecutively with MTBE (250 mL), MeOH (2250 mL), water (2300 mL), MeOH (2*250 mL) and MTBE (250 mL) and dried to give an off-white solid as product (304.2 g, 88percent yield). | 88% |
| Stage #1: 9,9-dimethylxanthene With N,N,N,N,-tetramethylethylenediamine; sec.-butyllithium In tert-butyl methyl ether; cyclohexane at 10 – 20℃ for 16h Stage #2: chloro-diphenylphosphine In cyclohexane; water at 20℃ for 5.75h Experimental Procedure Preparation of 9,9-dimethyl-4,5-bis(diphenylphosphino)xanthene, Xantphos To a 1L round bottom flask (RBF) are added MTBE (500 mL), 9,9-dimethylxanthene (26.65 g) and TMEDA (30.6 g). After degassing the solution, s-BuLi (155 g, 1.3 M in cyclohexane) is cannulated into a dropping funnel and then slowly added over 30 min while maintaining the batch temperature at 10-20° C. The mixture is then aged for 16 h at room temperature. Ph2PCl is added slowly via a dropping funnel while maintain the mildly exothermic reaction at 10-20° C. ~60percent of the Ph2PCl (30 mL) is added in 0.5 hour. The mixture is aged for 15 minutes before addition of the remaining Ph2PCl. After aged for 5.5 h at room temperature, the reaction is quenched with MeOH (2.0 mL). The product is filtered and the slightly yellow solid is washed consecutively with MeOH (200 mL), water (200 mL), MeOH (200 mL) and MTBE (200 mL) and dried to give an off-white solid as product (54.8 g, 77percent yield). | 77% |
| Stage #1: 9,9-dimethylxanthene With N,N,N,N,-tetramethylethylenediamine In diethyl ether for 1h Stage #2: With sec.-butyllithium In diethyl ether at 20℃ for 15h Stage #3: chloro-diphenylphosphine In diethyl ether; hexane for 15h | 74% |
| With n-butyllithium; N,N,N,N,-tetramethylethylenediamine 1.) hexane, heptane, reflux, 10 min; 2.) hexane, heptane, 0 deg C Yield given. Multistep reaction | |
| Stage #1: 9,9-dimethylxanthene With N,N,N,N,-tetramethylethylenediamine; sec.-butyllithium In diethyl ether; hexane at 20℃ Inert atmosphere Stage #2: chloro-diphenylphosphine In diethyl ether; hexane Inert atmosphere | |
| Stage #1: 9,9-dimethylxanthene With N,N,N,N,-tetramethylethylenediamine; sec.-butyllithium In tert-butyl methyl ether; cyclohexane at 10 – 20℃ for 16h Stage #2: chloro-diphenylphosphine In tert-butyl methyl ether; cyclohexane at 20℃ for 5.5h | |
| Stage #1: 9,9-dimethylxanthene With n-butyllithium; N,N,N,N,-tetramethylethylenediamine In tetrahydrofuran; hexane at 20℃ for 16h Stage #2: chloro-diphenylphosphine In tetrahydrofuran; hexane at 0℃ for 16h regioselective reaction |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315: Causes skin irritation [Warning Skin corrosion/irritation] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335: May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Room temperature, sealed, away from light and filled with nitrogen gas to prevent oxidation | |
| HS Code | 294200 |
| Storage | Room temperature, sealed, away from light and filled with nitrogen gas to prevent oxidation |
| Shelf Life | 2 years |
| Market Price | USD 900/kg |
| Use Pattern |
| 4,5-Bis(diphenylphosphino)-9,9-dimethylxanthene CAS#: 161265-03-8 is a phosphine ligand |
| conversion of the tetracycline reactive intermediate to the carboxaldehyde substituted tetracycline compounds |
| bidentate organophosphorus ligand for catalytic system for the hydrocyanation of unsaturated compounds |
| Ligand for catalyst system for preparation of dinitriles by hydrocyanation |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Johnson Matthey | https://matthey.com/ |
| Warshel Chemical Ltd | https://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |