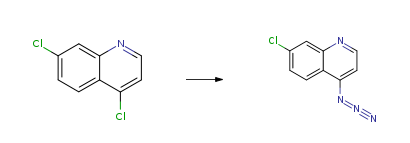
4,7-Dichloroquinoline CAS#: 86-98-6; ChemWhat Code: 32612
Identification
| Product Name | 4,7-Dichloroquinoline |
| IUPAC Name | 4,7-dichloroquinoline |
| Molecular Structure | 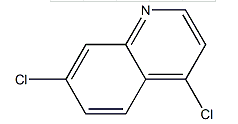 |
| CAS Registry Number | 86-98-6 |
| EINECS Number | 201-714-7 |
| MDL Number | MFCD00006774 |
| Beilstein Registry Number | 125359 |
| Synonyms | 4,7-dichloroquinoline, 4,7-dichloro-quinoline, 4,7‐dichloroquinoline, 4,7-dicholoroquinoline, 4,7-Dichloroquinoline, 4,7-dichloroquinolne, 4,7-dichlorquinoline;4,7-Dichloroquinoline CAS#: 86-98-6 |
| Molecular Formula | C9H5Cl2N |
| Molecular Weight | 198.05 |
| InChI | InChI=1S/C9H5Cl2N/c10-6-1-2-7-8(11)3-4-12-9(7)5-6/h1-5H |
| InChI Key | HXEWMTXDBOQQKO-UHFFFAOYSA-N |
| Canonical SMILES | c1cc2c(ccnc2cc1Cl)Cl |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN109232417 | 4 – Phenoxy quinoline compound of preparation method (by machine translation) | 2019 |
| CN109608394 | Nitrogen hetero-aromatic amines for synthesizing the compounds and nitrogen hetero-aromatic amine compound (by machine translation) | 2019 |
| CN108503582 | 2 – (1 H) – quinoline compound of microwave-assisted synthesis method (by machine translation) | 2018 |
| EP1746096 | 2-Arylbenzothiazole analogues and uses thereof in the treatment of cancer | 2007 |
| US5939568 | Accelerated catalysis of olefinic epoxidations | 1999 |
Physical Data
| Appearance | White to yellow powder |
| Solubility | chloroform: soluble50mg/mL, clear, colorless to greenish-yellow |
| Flash Point | 164 °C |
| Refractive index | 1.6300 (estimate) |
| Melting Point, °C | Solvent (Melting Point) |
| 83 – 84 | |
| 140 – 142 | ethanol |
| 86.9 | |
| 93 | |
| 86 – 87 | |
| 93 | petroleum ether |
| 84.5 – 85 | aq. ethanol |
| 83.5 – 84.5 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 148 | 10 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Association with compound | dimethylsulfoxide, various solvent(s) | 25 | ferriprotoporphyrin IX |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | 300 |
| Chemical shifts | 13C | chloroform-d1 | 75 |
| Spectrum | 1H | chloroform-d1 | 200 |
| Spectrum | 13C | chloroform-d1 | 50 |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 300 |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 1H | acetone-d6 | 400 |
| Chemical shifts | 1H | CDCl3 | 400 |
| Chemical shifts | 13C | CDCl3 | |
| NMR | |||
| NMR with shift reagents |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | potassium bromide |
| Bands | KBr |
| Description (Mass Spectrometry) |
| electrospray ionisation (ESI), spectrum |
| electron impact (EI), spectrum |
| spectrum |
| EI (Electron impact), Spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Log epsilon |
| methanol | 228 | 3.8 | ||
| Spectrum | H2O, ethanol | 260 – 360 nm | ||
| UV/VIS | ||||
| Absorption maxima | CHCl3 | 279, 310, 324 |
| Description (Phosphorescence Spectroscopy) | Comment (Phosphorescence Spectroscopy) |
| Maxima | 489 nm, 500 nm |
Route of Synthesis (ROS)
| Conditions | Yield |
| With sodium azide In N,N-dimethyl-formamide at 65℃; for 6h; | 95% |
| With sodium azide In dimethyl sulfoxide | 95% |
| With sodium azide In ethanol; water for 2h; Heating; | 94% |
| With sodium azide In N,N-dimethyl-formamide at 20 – 85℃; for 8h; Molecular sieve; | 91% |
| With sodium azide; sodium iodide In water; N,N-dimethyl-formamide at 80℃; for 8h; | 91% |
| With sodium azide In N,N-dimethyl-formamide at 85℃; for 8h; Molecular sieve; | 91% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315 (93.1%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (79.31%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (77.59%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the?GHS Classification?page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | 293349 |
| Storage | Protected from light and humidity in a clean place prefer temperature below 30℃. |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 198.051 |
| logP | 3.369 |
| HBA | 1 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 12.89 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (quant) | Unit |
| 5.58 | inhibition rate(Biofilm Formation) | 122 | % |
| 5.18 | inhibition rate(Biofilm Formation) | 101 | % |
| 4.88 | inhibition rate(Biofilm Formation) | 170 | % |
| 4.7 | IC50 | 20 | μM |
| 4.7 | IC50 | 20 | μM |
| 3.49 | MIC | 64 | μg/ml |
| 2.6 | MIC | 2525 | μM |
| 1 | Activity(Inhibition) | ||
| log K | 2.5 |
| Quantitative Results | ||
| 1 of 7 | Target | Receptor-type tyrosine-protein kinase FLT3 [human]:Wild |
| Substance action on target | Inhibitor | |
| Biological material | human | |
| Assay Description | Inhibitory activity of the compound against human FLT3 (5 nM) using 5 uM ATP, 3 uM polyEY upon incubation at ambient temperature for 3 hours in 20 mM Tris-HCl, pH 7.5 using luciferase-coupled chemiluminescence kinase assay | |
| 2 of 7 | Target | Vascular endothelial growth factor receptor 2 [human]:Wild |
| Biological material | human | |
| Assay Description | Inhibitory activity of the compound against human KDR (5 nM) using 3 uM ATP, 1.6 uM polyEY upon incubation at ambient temperature for 4 hours in 20 mM Tris-HCl, pH 7.5 using luciferase-coupled chemiluminescence kinase assay | |
| 3 of 7 | Target | Vascular endothelial growth factor receptor 3 [human]:Wild |
| Biological material | human | |
| Assay Description | Inhibitory activity against human FLT-4 (1 nM) upon incubation at ambient temperature for 1 hour in 75 mM Hepes, pH 7.4 using 5 uM ATP, 3 nM biotinylated poly (Glu, Tyr) | |
| 4 of 7 | Target | Hepatocyte growth factor receptor [human]:Wild |
| Biological material | human | |
| Assay Description | Inhibitory activity of the compound against human c-Met (10 nM) using 1 uM ATP, 1 uM polyEY upon incubation at ambient temperature for 2 hours in 20 mM Tris-HCl, pH 7.5 using luciferase-coupled chemiluminescence kinase assay | |
| 5 of 7 | Target | Mast/stem cell growth factor receptor Kit:Wild |
| Substance action on target | Inhibitor | |
| Assay Description | Inhibitory activity against c-KIT upon incubation at ambient temperature for 1 hour in 75 mM Hepes, pH 7.4 using ATP, biotinylated poly (Glu, Tyr) | |
| 6 of 7 | Effect | antifungal agent |
| Assay Description | Target : Candida albicans MC303 Bioassay : cells were prepeared; cells were incubated in inducung media with title compound at 37°C for 24h; cells were observed microscopically; type5 – long-hyphal (WT); 1 – non-hyphal; 2-4 intemidiate states; compounds that result in phenotype 1 or 2 are considered to have significant anti-invasin properties | |
| Results | Phenotype 5 | |
| 7 of 7 | Effect | antifungal agent |
| Biological material | Candida albicans | |
| Results | overnight growth inhibition 0.95% |
| Use Pattern |
| 4,7-Dichloroquinoline CAS#: 86-98-6 as Pharmaceuticals |
| 4,7-Dichloroquinoline CAS#: 86-98-6 as synthesis antimalarial drug intermediate |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |