5-Hydroxymethylfurfural CAS#: 67-47-0; ChemWhat Code: 73629
Identification
| Product Name | 5-Hydroxymethylfurfural |
| IUPAC Name | 5-(hydroxymethyl)furan-2-carbaldehyde |
| Molecular Structure | 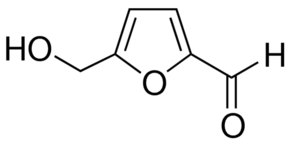 |
| CAS Registry Number | 67-47-0 |
| EINECS Number | 200-654-9 |
| MDL Number | MFCD00003234 |
| Beilstein Registry Number | 110889 |
| Flavis Number | 13.139 |
| Synonyms | 5-hydroxymethyl-2-furfuraldehyde, 5-hydroxymethylfurfural, 5-HMF |
| Molecular Formula | C6H6O3 |
| Molecular Weight | 126.112 |
| InChI | InChI=1S/C6H6O3/c7-3-5-1-2-6(4-8)9-5/h1-3,8H,4H2 |
| InChI Key | NOEGNKMFWQHSLB-UHFFFAOYSA-N |
| Canonical SMILES | C1=C(OC(=C1)C=O)CO |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US9938548 | Process for preparing (meth)acrylic esters of functionalized furyl alcohols | 2018 |
| JP2018/193353 | The furan derivative | 2018 |
| JP6168044 | Tetrahydrofuran compound | 2017 |
| JP2016/37486 | Method of manufacturing hydroxytetrahydrofuran compd. (by machine translation) | 2016 |
| US2016/339414 | PROCESS FOR THE PREPARATION OF 2, 5-DIMETHYLEFURAN AND FURFURYL ALCOHOL OVER RUTHENIUM SUPPORTED CATALYSTS | 2016 |
| WO2016/195115 | SUPPORTED CATALYST FOR ALDEHYDE COUPLING REACTION, METHOD FOR PERFORMING ALDEHYDE COUPLING REACTION, AND METHOD FOR REGENERATING SUPPORTED CATALYST FOR ALDEHYDE COUPLING REACTION | 2016 |
Physical Data
| Appearance | Yellow or off-yellow solid |
| Melting Point | 28-34 °C |
| Flash Point | 79 °C |
| Solubility | Soluble in water, alcohol, ethyl acetate, acetone, dimethylformamide, benzene, ether and chloroform. |
| Sensitivity | Air & Light Sensitive |
| Stability | Light Sensitive, Very Hygroscopic |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 114-116 | 1 |
| 110 | 0.02 |
| 90 | 0.0225023 |
| 85 – 88 | 0.01 |
| 120 – 125 | 0.3 |
| 154 – 155 | 12 |
| 140 – 142 | 7 |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C |
| 1.56 | 589 | 20 |
| 1.559 | 589 | 25 |
| 1.5627 | 589 | 18 |
| 1.5533 | 589 | 25 |
| 1.552 | 656.3 | 39 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.262 | 25 | 25 |
| 1.2062 | 4 | 25 |
| 1.268 | 15 | 15 |
| 1.2629 | 4 | 36 |
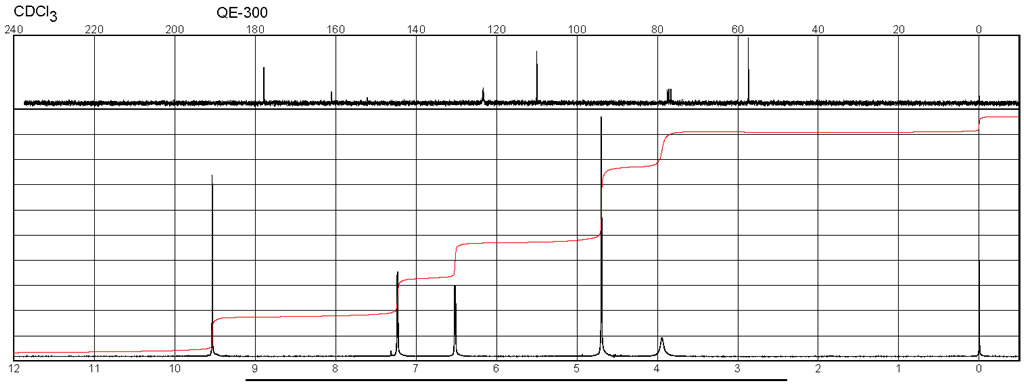
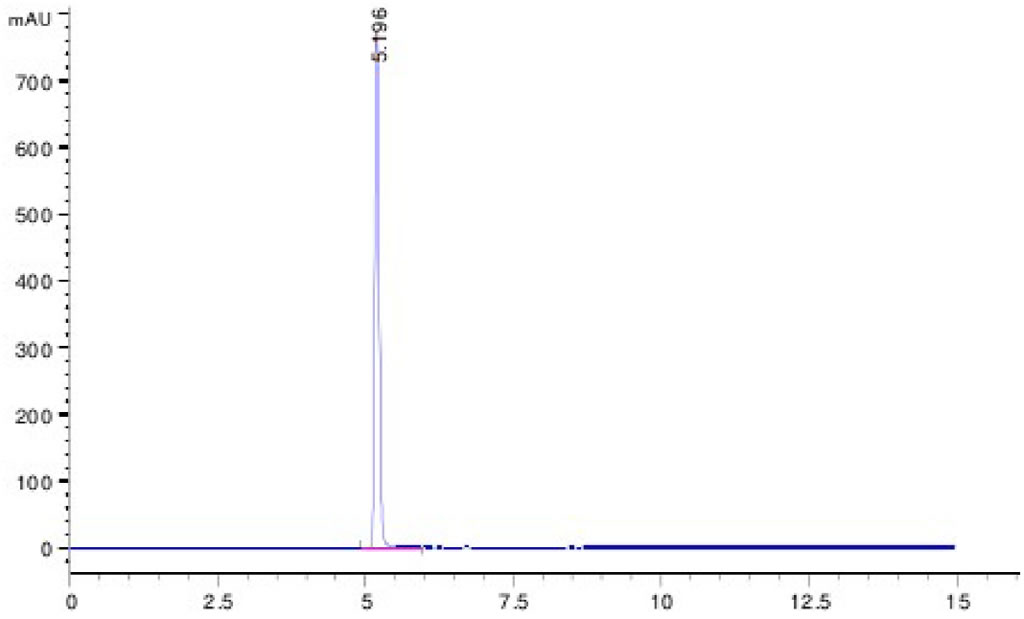
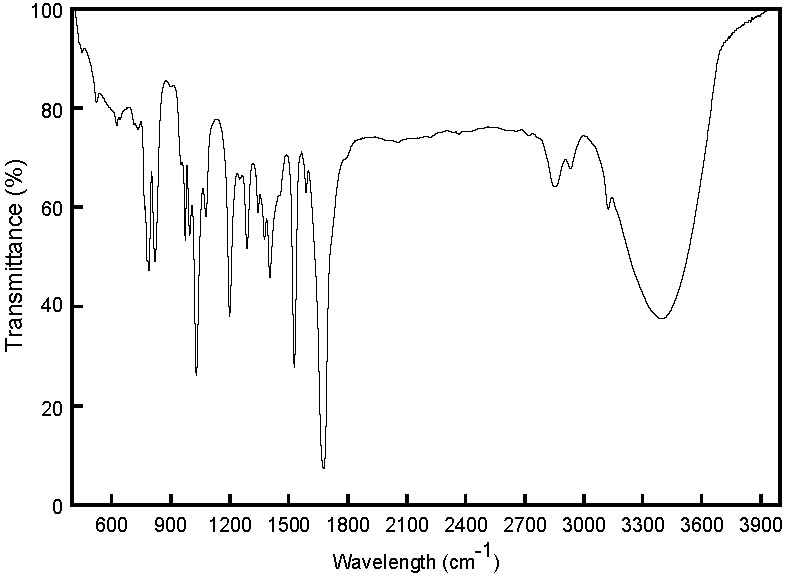
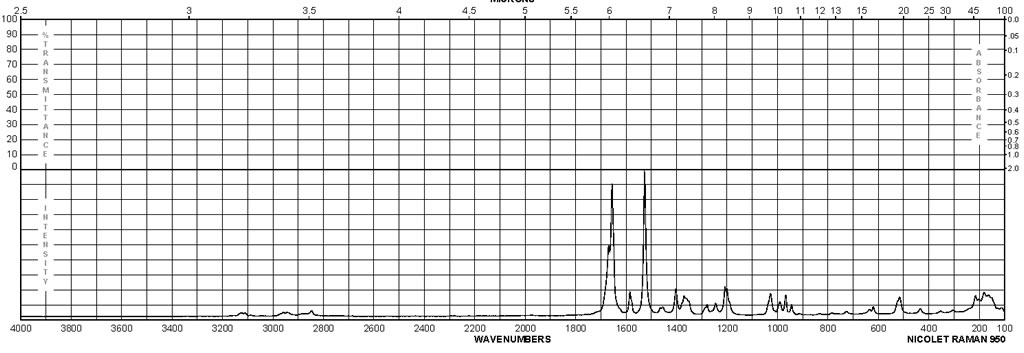
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | 25 | 500 |
| Chemical shifts | 13C | chloroform-d1 | 25 | 125 |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | water | 284 |
| Absorption maxima | ethanol | 278 |
| Absorption maxima | methanol | 221, 281 |
Route of Synthesis (ROS)
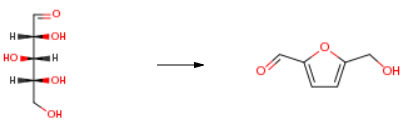
| Conditions | Yield |
| With phosphoric acid; potassium dihydrogen phosphate In water; iso-butanol at 143℃; under 4500.45 Torr; for 3h; Concentration; Temperature; Reagent/catalyst; Inert atmosphere; Experimental Procedure reaction was carried out in the same manner as in Example 1 except that 1.501 g of D (+) xylose (10.0 mmol) was used as the sugar raw material and the reaction time was changed to 3.0 hours.After the reaction, the 2-butanol layer was transferred to a 100 ml Erlenmeyer flask, and the aqueous layer was washed three times with 10 ml of 2-butanol, and these washing solutions were collected in the flask.1.0 ml of diethylene glycol diethyl ether (internal standard substance for GC analysis) and 1 g of calcium hydroxide (for removing residual acid) were added to the recovered 2-butanol solution, and then mixed.The mixture was filtered through 2 C filter paper, and the filtrate was subjected to GC analysis to determine the amount of 2-furaldehyde (FAL) produced.The results are shown in Table 6. | 75% |
| With chromium dichloride; N-benzyl-N,N,N-triethylammonium chloride In ethylene glycol at 100℃; for 0.5h; Experimental Procedure Organic ammonium salt and diethylene glycol solvent were mixed, and various carbohydrates were converted to HMF using catalyst.[0038]Choline chloride (Example 6-1 and 6-2) or benzyltriethylammonium chloride (BTEAC, used in Example 6-3) was used as organic ammonium salt, diethylene glycol was used as hydroxyl organic solvent, CrCl2 (Example 6-1 and 6-3) or CrCl3.6H2O (Example 6-2) was used as catalyst, and sucrose, starch or xylose was used as carbohydrate in Example 6. Organic ammonium salt was mixed with diethylene glycol solvent at the ratio 75 mol percent and heated to 100° C. to form a solution. The solution was mixed with sucrose, starch or xylose, and 6 mol percent catalyst (with amount based on the mole of the carbohydrate) was added. The reactions were conducted at the temperature of 100° C., for sucrose, 120° C. for starch, and 100° C. for xylose, respectively. And sucrose, starch, or xylose was converted to the product HMF. The experimental conditions and the experimental results of HMF yields in the Example 6 are listed in Table 7. The yield of HMF can reach 69percent in Example 6-1. | 51% |
| With SiO2-MgCl2 composite In acetonitrile at 180℃; for 1.5h; Autoclave; | 17% |
| With Aluminosilicate beta zeolite calcined at 500 °C In tetrahydrofuran; water; dimethyl sulfoxide at 180℃; for 3h; Time; Autoclave; | |
| With sulfonated graphene quantum dots In water; dimethyl sulfoxide at 170℃; for 2h; |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H315: Causes skin irritation [Warning Skin corrosion/irritation] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335: May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] H412: Harmful to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P271, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 293219 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD 380/kg |
| Use Pattern |
| hair dye cell growth promoter and hair dermal papilla cell growth promoter |
| Cosmetics/dental/toilet |
| enhanced neuronal function involving elevated levels of pCREB and/or Egr1 |
| the preparation of 2,5-furandicarboxylic acid and esters thereof |
| in certain dried fruits and caramel products, juces from dried plums |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Avantium | https://www.avantium.com/ |
| Ulcho Biochemical Ltd | https://www.ulcho.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |