6-Hexanolactone CAS#: 502-44-3; ChemWhat Code: 51930
Identification
| Product Name | 6-Hexanolactone |
| IUPAC Name | oxepan-2-one |
| Molecular Structure | 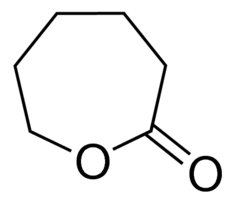 |
| CAS Registry Number | 502-44-3 |
| EINECS Number | 207-938-1 |
| MDL Number | MFCD00003267 |
| Beilstein Registry Number | 106919 |
| Synonyms | hexahydro-2H-oxepin-2-one; ε-caprolactone; Hexanoic acid, 6-hydroxy-, lactone;Hexanoic acid, 6-hydroxy-, ε-lactone |
| Molecular Formula | C6H10O2 |
| Molecular Weight | 114.142 |
| InChI | InChI=1S/C6H10O2/c7-6-4-2-1-3-5-8-6/h1-5H2 |
| InChI Key | PAPBSGBWRJIAAV-UHFFFAOYSA-N |
| Canonical SMILES | C1CCC(=O)OCC1 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| JP2019/43923 | The method of making a composition derived from the ester compound (by machine translation) | 2019 |
| WO2015/67657 | PROCESS FOR THE OXIDATION OF ORGANIC CARBONYL COMPOUNDS | 2015 |
| JP2015/227317 | Post processing is performed using a tin GRK · substd. zeolite oxidation ester ketone (by machine translation) | 2015 |
| WO2006/20234 | CONVERSION OF A MULTIHYDROXYLATED-ALIPHATIC HYDROCARBON OR ESTER THEREOF TO A CHLOROHYDRIN | 2006 |
Physical Data
| Appearance | Transparent, colorless or pale yellow liquid |
| Water Solubility | >1000 MG/L (20 ºC) |
| Water Solubility | 2 g/100 mL (25 oC) |
| Melting Point, °C | Solvent (Melting Point) |
| -1.02 | |
| 5 | |
| -1.3 | |
| 0 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 233 – 235 | 760 |
| 131 | |
| 150 – 155 | 7.50075 |
| 74 – 75 | 0.5 |
| 39 – 41 | 0.7 |
| 50 – 52.6 | 0.1 |
| 118 – 120 | 15 |
| 98 – 100 | 9 |
| Refractive Index | Wavelength (Refractive Index), nm | Temperature (Refractive Index), °C |
| 1.45583 – 1.46361 | 589 | 20 – 40 |
| 1.4612 – 1.4615 | 589 | 25 |
| 1.4635 | 589 | 20 |
| 1.4608 | 589 | 30 |
| 1.4605 | 589 | 20 |
| 1.4608 | 589 | 24 |
| 1.4481 | 589 | 24 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 0.999984 | 4.99 |
| 1.00125 | 4.99 |
| 1.00375 | 4.99 |
| 0.999715 | 9.99 |
| 0.99911 | 14.99 |
| 1.00024 | 14.99 |
| 0.998211 | 19.99 |
| 0.997047 | 24.99 |
| 0.99403 | 34.99 |
| 0.990211 | 44.99 |
| 1.0693 | 20 |
| 1.0698 | 24 |
| 1.071 | 25 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| NMR spectrum of the complex | D2O | alpha cyclodextrin | |
| NMR spectrum of the complex | D2O | β-cyclodextrin | |
| Stability constant of the complex with … | CCl4 | 25 | 4-Fluorophenol |
| Stability constant of the complex with … | CCl4 | 25 | chloranil |
| UV/VIS spectrum of the complex | CCl4 | 25 | chloranil |
| Stability constant of the complex with … | CCl4 | 25 | 2,3,5,6-tetrafluoro-1,4-benzoquinone |
| UV/VIS spectrum of the complex | CCl4 | 25 | 2,3,5,6-tetrafluoro-1,4-benzoquinone |
| Description (Electrical Moment) | Moment (Electrical Moment), D | Method (Electrical Moment) | Solvent (Electrical Moment) |
| Dipole moment | 4.45 | Dielectric constant (ε) | benzene |
| Enthalpy of Vaporization, Jmol-1 | Temperature (Enthalpy of Vaporization), °C | Comment (Enthalpy of Vaporization) |
| Enthalpy of vaporization given | ||
| 62006.5 | 24.9 |
| Heat Capacity Cp0, Jmol-1K-1 | Temperature (Heat Capacity Cp0), °C |
| 5.08 – 186.1 | -258.9 – -11.4 |
| 187.6 – 207 | -3.3 – 54.6 |
| Description (Liquid/Solid Systems (MCS)) | Temperature (Liquid/Solid Systems (MCS)), °C | Partner (Liquid/Solid Systems (MCS)) |
| Liquid-solid phase equilibrium | -28.15 – 6.85 | benzene |
| Liquid-solid phase equilibrium | -63.15 – 1.85 | toluene |
| Liquid-solid phase equilibrium | -13.15 – 6.85 | cyclohexane |
| Liquid-solid phase equilibrium | -53.15 – 6.85 | propan-1-ol |
| Liquid-solid phase equilibrium | -73.15 – 6.85 | methanol |
| Liquid-solid phase equilibrium | -23.15 – 6.85 | water |
| Liquid-solid phase equilibrium | -83.15 – 6.85 | 2-Pentanone |
| Description (Liquid/Vapour Systems (MCS)) | Temperature (Liquid/Vapour Systems (MCS)), °C | Partner (Liquid/Vapour Systems (MCS)) |
| Activity coefficients of the components in the mixture | 30 – 60 | dichloromethane |
| Activity coefficients of the components in the mixture | 30 – 60 | chloroform |
| Activity coefficients of the components in the mixture | 30 – 60 | acetone |
| Activity coefficients of the components in the mixture | 30 – 60 | isopropyl alcohol |
| Activity coefficients of the components in the mixture | 30 – 60 | methanol |
| Activity coefficients of the components in the mixture | 30 – 60 | n-heptane |
| Activity coefficients of the components in the mixture | 30 – 60 | propan-1-ol |
| Activity coefficients of the components in the mixture | 30 – 60 | tetrahydrofuran |
| Activity coefficients of the components in the mixture | 30 – 60 | vinyl acetate |
| Activity coefficients of the components in the mixture | 30 – 60 | tert-Amyl methyl ether |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | |
| Chemical shifts | 1H | chloroform-d1 | 300 |
| Chemical shifts | 1H | chloroform-d1 | 500 |
| Chemical shifts | 13C | ||
| Chemical shifts | 13C | chloroform-d1 | 100 |
| Chemical shifts | 13C | chloroform-d1 | 600 |
| COSY (Correlation Spectroscopy) | 1H, 1H | chloroform-d1 | 500 |
| HMQC (Heteronuclear Multiple Quantum Coherence), Spectrum | 1H, 13C | chloroform-d1 | |
| Spectrum | 1H | benzene-d6 | 300 |
| Chemical shifts | 1H | CDCl3 | 400 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Original Text (IR Spectroscopy) | Comment (IR Spectroscopy) | Signals, cm-1 |
| ATR (attenuated total reflectance), Bands | IR (ATR)/cm-1 2988, 2901, 1726 | 2988, 2901, 1726 | ||
| Bands | potassium bromide | film | ||
| Bands | neat (no solvent) | 1730 cm**(-1) | ||
| Bands | 1818 – 1000 cm**(-1), Film. |
Route of Synthesis (ROS)
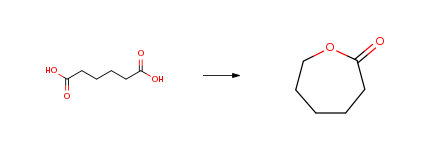
| Conditions | Yield |
| With hydrogen In water at 375℃; for 5h; Experimental Procedure Preparation of Caprolactone [0243] 10 ml of catalyst 4 reduced beforehand at 450° C. and 5 ml of glass powder used as static mixer vaporizer are placed in a vertical glass reactor 22 mm in diameter. [0244] The catalytic bed is heated under a stream of 5 l/h of hydrogen to 375° C. [0245] After stabilizing the catalytic bed under these conditions for 30 minutes, injection onto the catalytic bed, by means of a syringe pump, of an aqueous adipic acid solution at 2percent w/w at a flow rate of 10 ml/h is commenced. [0246] The reaction gas stream is then condensed in a receiver immersed in an ice-water bath. [0247] After injection for 5 hours under these conditions, the condensates are analyzed by GC. [0248] For a degree of conversion of 100percent, an 85percent yield of caprolactone is obtained. | 85% |
| With hydrogen; Ru4(CO)82(PBu3)4 In diethyl ether; toluene at 180℃; under 98800 Torr; for 48h; Yield given; | |
| With hydrogen In 1,4-dioxane at 265℃; under 18751.9 Torr; Experimental Procedure Production of ε-caprolactone from the adipic acid through the hydrogenation reaction [catalyst: CuO(80)SiO2 (20)]The catalyst was produced by using the same method as Example 1. The reaction temperature was fixed to 265°C, the reaction was performed by using the same method as Example 1, and the reaction results over time are described in the following Table 7. The present catalyst exhibited high selectivity of 86.5percent of ε-caprolactone. |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P264, P280, P305+P351+P338, and P337+P313 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 293220 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD 4.2/kg |
| Use Pattern |
| Pharmaceuticals |
| 6-Hexanolactone CAS#: 502-44-3 can be used for polymers/polymer applications |
| producing a lactone copolymer in combination with a second monomer which is used in bio-plastics |
| producing a lactone copolymer in combination with a second monomer which is used in compositions for 3D printing |
| producing a lactone copolymer in combination with a second monomer which is used in hot melt adhesives |
| producing a lactone copolymer in combination with a second monomer which is used in thermoplastics |
| 6-Hexanolactone CAS#: 502-44-3 can be a monomer for producing biodegradable polymer backbone suitable for manufacturing antimicrobial releasing polymers |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |