[6,6]-phenyl C61butyric acid methyl ester CAS#: 160848-22-6; ChemWhat Code: 57496
Identification
| Product Name | [6,6]-phenyl C61butyric acid methyl ester |
| Molecular Structure | ![[6,6]-phenyl C61butyric acid methyl ester CAS 160848-22-6](https://www.chemwhat.com/wp-content/uploads/2017/11/66-phenyl-C61butyric-acid-methyl-ester-CAS-160848-22-6.png) |
| CAS Registry Number | 160848-22-6 |
| MDL Number | MFCD07784544 |
| Synonyms | [6,6]-phenyl C61butyric acid methyl ester, 1-<3-(Methoxycarbonyl)propyl>-1-phenyl-<6,6>-C61, methanofullerene [6,6]-phenyl C61-butyric acid methyl ester, metanofullerene[6,6]-phenyl C61-butyric acid methyl ester, 1-(3-(Methoxycarbonyl)propyl)-1-phenyl<6.6>C61, (6,6)-phenyl-C61-butyric acid methyl ester, [6,6]-phenyl C61butyric acid methyl ester,PCBM,PC61BM |
| Molecular Formula | C72H14O2 |
| Molecular Weight | 910.902 |
| InChI | InChI=1S/C72H14O2/c1-74-11(73)8-5-9-70(10-6-3-2-4-7-10)71-66-59-52-40-32-23-14-12-13-15-18(14)27-34(32)42-43-35(27)33-24(15)26-22-17(13)20-19-16(12)21-25(23)38(40)46-44-30(21)28(19)36-37-29(20)31(22)45-47-39(26)41(33)53-55(43)64(63(66)54(42)52)67-60(53)58(47)62-51(45)49(37)56-48(36)50(44)61(57(46)59)68(71)65(56)69(62)72(67,70)71/h2-4,6-7H,5,8-9H2,1H3 |
| InChI Key | MCEWYIDBDVPMES-UHFFFAOYSA-N |
| Canonical SMILES | COC(=O)CCCC1(C23C14C5=C6C7=C8C5=C9C1=C5C%10=C%11C%12=C%13C%10=C%10C1=C8C1=C%10C8=C%10C%14=C%15C%16=C%17C(=C%12C%12=C%17C%17=C%18C%16=C%16C%15=C%15C%10=C1C7=C%15C1=C%16C(=C%18C7=C2C2=C%10C(=C5C9=C42)C%11=C%12C%10=C%177)C3=C16)C%14=C%138)C1=CC=CC=C1 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2017/40543 | ORGANIC SOLAR CELL AND MANUFACTURING METHOD THEREFOR | 2017 |
| CN106800511 | A fullerene derivative and the perovskite in the solar cell application (by machine translation) | 2017 |
| US2011/130588 | COMPOSITION, AND ORGANIC PHOTOELECTRIC CONVERSION ELEMENT COMPRISING SAME | 2011 |
| WO2008/6071 | BLENDS OF FULLERENE DERIVATIVES, AND USES THEREOF IN ELECTRONIC DEVICES | 2008 |
Physical Data
| Appearance | Dark Brown Powder |
| Solubility | chlorobenzene: soluble organic solvents: soluble toluene: soluble |
| Orbital Energy | HOMO 6.1 eV LUMO 3.7 eV |
| Semiconductor Properties | N-type (mobility=0.21 cm2/V·s) |
| Melting Point, °C |
| 290 |
| 256 |
| 287 |
| 285 |
| Sublimation, °C |
| 320 |
| 520 |
| 560 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.613 | |
| 1.621 | 24.84 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Partner (Association (MCS)) |
| NMR spectrum of the complex | Poly(3-hexylthiophene-2,5-diyl) | |
| UV/VIS spectrum of the complex | film | poly(3-hexylthiophene), regioregular, Mw 87 kg/mol |
| IR spectrum of the complex | KBr | poly[1-(N-carbazolyl)-penta-1,3-diyne-5-ol], solid state UV polymerization; Monomer(s): 1-(N-carbazolyl)-penta-1,3-diyne-5-ol |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | 300 |
| Chemical shifts | 13C | chloroform-d1 | 75 |
| Chemical shifts | 1H | CDCl3 | 400 |
| Chemical shifts | 13C | CS2 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C | Comment (IR Spectroscopy) |
| Spectrum | polymer matrix | 22.84 – 119.84 | excited state. Object(s) of Study: time dependence. Object(s) of Study: concentration dependence |
| Bands | Kbr | 1737 – 527 cm**(-1) |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | toluene | 331, 435, 700 | |
| Band assignment, Spectrum | 1,2-dichloro-benzene | 330 | |
| Spectrum | chloroform | 260, 329, 431 | |
| Spectrum | tetrahydrofuran | 258, 328 | |
| Spectrum | dimethylsulfoxide, H2O | 290 – 700 nm |
Route of Synthesis (ROS)
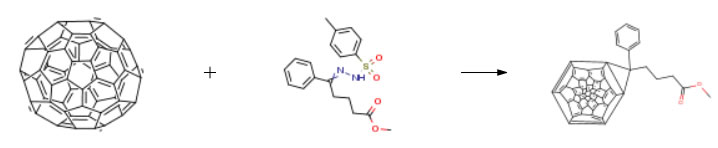
| Conditions | Yield |
| With pyridine; sodium methylate In 1,2-dichloro-benzene at 180℃ for 1h Concentration Microwave irradiation | 65% |
| With pyridine; sodium methylate In 1,2-dichloro-benzene at 65 – 70℃ for 24h | 35% |
| Stage #1: 5-phenyl-5-(p-toluenesulfonylhydrazide)methyl pentanoate With pyridine; sodium methylate at 75℃ for 0.25h Inert atmosphere Stage #2: fullerene-C60 In 1,2-dichloro-benzene at 180 – 200℃ for 20h Inert atmosphere Experimental Procedure 250mL three-neck flask was added 1.57 g of (4.2 × 10-3mol) a14,0.23g (4.2 × 10-3mol) of sodium methoxide and 50g of pyridine.Magnetic stirring, is heated under a nitrogen atmosphere was heated to 75 , incubation after 15min, was added 1.5 g (2.1 × 10-3mol) C60And 60g of o-dichlorobenzene (ODCB), continuing the reaction at this temperature for 20h.Completion of the reaction, cooled to room temperature, the pressure to remove the solvent to give a brown solid 3.1g.Chromatography column purification to give 0.67g brown solid.Above 0.67g brown solid was added 50g of o-dichlorobenzene was dissolved, with magnetic stirring, heated to reflux for 18 h, the solvent removed under reduced pressure, purified by column chromatography to give 0.67g brown solid in 35percent yield. | 35% |
| Stage #1: fullerene-C60; 5-phenyl-5-(p-toluenesulfonylhydrazide)methyl pentanoate In 1,2-dichloro-benzene at 70℃ Stage #2: In 1,2-dichloro-benzene Heating | |
| Stage #1: 5-phenyl-5-(p-toluenesulfonylhydrazide)methyl pentanoate With tetra(n-butyl)ammonium hydroxide In water; 1,2-dichloro-benzene at 20℃ for 0.5h Inert atmosphere Stage #2: fullerene-C60 In 1,2-dichloro-benzene at 110℃ for 2h Inert atmosphere Irradiation Experimental Procedure General procedure: Under Ar atmosphere, to a solution of tosylhydrazone (18.7 mg,0.050 mmol) in ODCB (2.0 mL) was added a solution of TBAH (1.0 M in H2O, 0.05 mL, 0.050 mmol) in H2O (1.95 mL), and themixture was stirred at r.t. for 0.5 h. A solution of C60 (36.0 mg,0.050 mmol) in ODCB was added to the mixture, and themixture was stirred (800–1000 rpm by magnetic stirrer),heated in an oil bath, and irradiated with a 375 W incandescentlamp for 2 h. During the irradiation, the oil bath temperaturewas kept at 105–115 °C by adjusting the distance between thelight source and the flask was approximately 15 cm. After thereaction, the layers were separated and the aqueous phase wasextracted with toluene (2 × 5 mL). The combined organic phasewas dried over MgSO4, filtered, and concentrated to 1 mL underreduced pressure. The residue was purified by silica gel columnchromatography [silica gel; 20 g, eluent; toluene for C60 andmonoadduct, toluene–CH2Cl2 (1:2) for bisadducts], and theobtained [6,6]PC61BM (1a) was dissolved in a small amount oftoluene and transferred to a 50 mL centrifuge tube. MeOH (ca.30 mL) was added, and the mixture was immersed in an ultrasoundbath for 1 min, the suspension was centrifuged (4000rpm, 30 min), the supernatant was decanted, and the residuewas treated with MeOH in the same manner. The product wasdried in vacuo at 60 °C. [6,6]PC61BM (1a, 23.5 mg, 0.026 mmol,52percent) was obtained as brown powder. Unreacted C60 (7.8 mg,0.011 mmol, 22percent) and bisadducts (10.9 mg, 0.010 mmol, 20percent)were isolated in the same manner. | |
| With pyridine; sodium methylate In 1,2-dichloro-benzene |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335: May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P261, P264, P271, P280, P304+P340, P305+P351+P338, P312, P337+P313, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Fill Nitrogen for long time, in container tightly sealed; Protect from light. | |
| HS Code | 294200 |
| Storage | Fill Nitrogen for long time, in container tightly sealed; Protect from light. |
| Shelf Life | 2 years |
| Market Price | USD 450/g |
| Use Pattern |
| [6,6]-phenyl C61butyric acid methyl ester CAS#: 160848-22-6 is often used as a soluble n-channel organic semiconductor; n-type layer in plastic electronic components, especially in bulk heterojunction OFET and photovoltaic cells (PV). |
| [6,6]-phenyl C61butyric acid methyl ester CAS#: 160848-22-6 has optical limiting properties in solution |
| [6,6]-phenyl C61butyric acid methyl ester CAS#: 160848-22-6 can be used as a soluble n-channel organic semiconductor. For use as an n-type layer in plastic electronics, especially bulk heterojunction OFETs and photovoltaic cells (PVs). |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Luminescence Technology Corp. | LT-S905PCBM |
| Warshel Chemical Ltd | https://www.warshel.com/methyl-66-phenyl-c61-butyrate-cas-160848-22-6/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |
