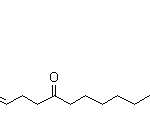
8,10-DODECADIEN-1-OL CAS#: 33956-49-9; ChemWhat Code: 80739
Identification
| Product Name | 8,10-DODECADIEN-1-OL |
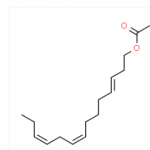
| IUPAC Name | (8E,10E)-dodeca-8,10-dien-1-ol |
| Molecular Structure | |
| CAS Registry Number | 33956-49-9 |
| Synonyms | (8E,10E)-dodeca-8,10-dienol;CAS Number: 33956-49-9; CAS NO:.33956-49-9 |
| Molecular Formula | C12H22O |
| Molecular Weight | 182.30 |
| InChI | InChI=1S/C12H22O/c1-2-3-4-5-6-7-8-9-10-11-12-13/h2-5,13H,6-12H2,1H3/b3-2+,5-4+ |
| InChI Key | CSWBSLXBXRFNST-MQQKCMAXSA-N |
| Canonical SMILES | C/C=C/C=C/CCCCCCCO |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2010/113837 | Production of pheromones and fragrances from substituted and unsubstituted 1-alken-3yl alkylates | 2010 |
| US2006/88563 | Use of spray adjuvant to enhance the movement of pesticides through plant canopies to the target | 2006 |
| US6838576 | Process for preparing functional group-containing olefinic compounds | 2005 |
Physical Data
| Appearance | Colorless or light yellow crystalline |
| Refractive index | 1.5050 (estimate) |
| Melting Point, °C | Solvent (Melting Point) |
| 29 – 30 | |
| 27 – 28.5 | |
| 31 – 32 | pentane |
| 28 – 29 | petroleum ether |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 98 – 101 | 0.5 |
| 90 – 92 | 0.001 |
| 120 – 122 | 0.5 |
| 120 – 121 | 1 |
| 122 | 0.5 |
| 105 | 0.1 |
| 80 – 90 | 0.01 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) |
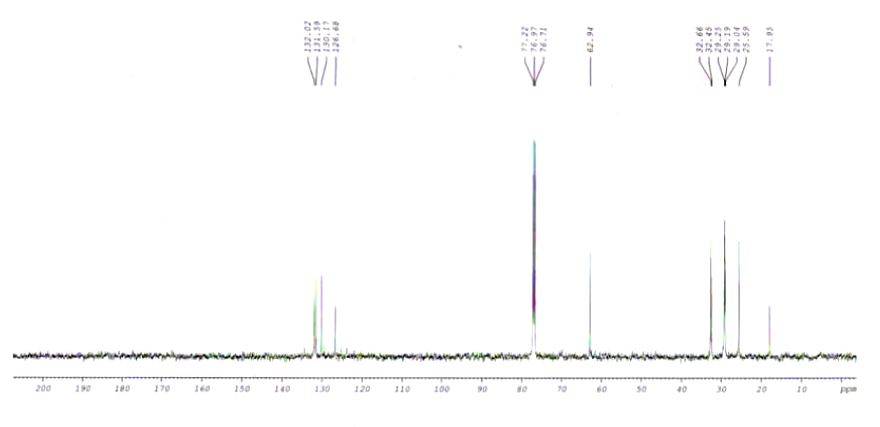
| Spectrum | 13C | |
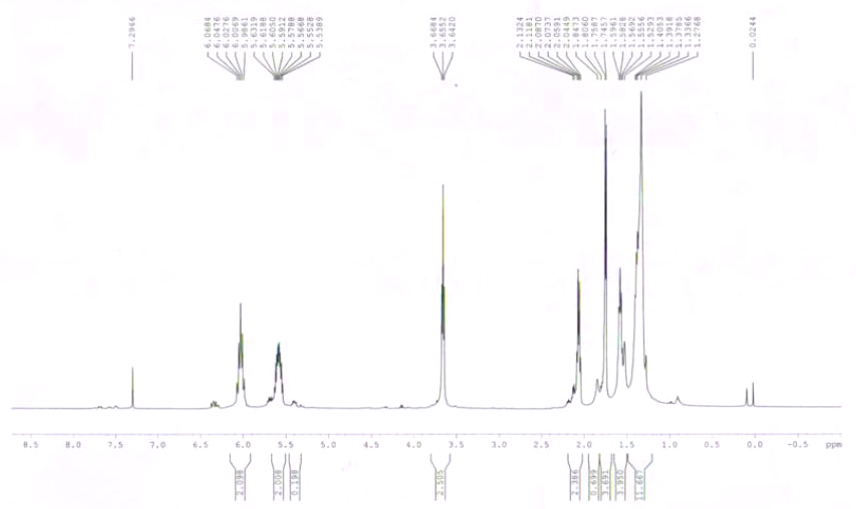
| Spectrum | 1H | |
| Chemical shifts | 1H | CDCl3 |
| Chemical shifts | 13C | CDCl3 |
| Chemical shifts | 1H | CCl4 |
| Chemical shifts | 1H | benzene-d6 |
| Chemical shifts | 13C | benzene-d6 |
| Spin-spin coupling constants | CDCl3 | |
| Spin-spin coupling constants | benzene-d6 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | neat (no solvent) | 3320 – 920 cm**(-1) |
| Bands | CHCl3 | 2930 – 990 cm**(-1) |
| Bands | neat (no solvent) | 3350 – 990 cm**(-1) |
| Bands | KBr | 3350 cm**(-1) |
| Bands | 3340 – 985 cm**(-1) | |
| IR |
| Description (Mass Spectrometry) |
| spectrum |
| spectrum, electron impact (EI) |
| spectrum, chemical ionization (CI) |
| fragmentation pattern, spectrum |
| electron impact (EI), spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | 205 – 300 nm | |||
| Absorption maxima | aq. ethanol | 229.9 | 28000 | |
| Absorption maxima | hexane | 229 | 22800 | |
| UV/VIS |
Route of Synthesis (ROS)
| Conditions | Yield |
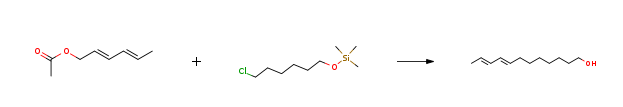
| Stage #1: (E,E)-sorbyl acetate; trimethylsilyl 6-chloro-1-hexyl ether With sodium In toluene at 0 – 80℃; for 12h; Inert atmosphere; Green chemistry; Stage #2: With sulfuric acid In water; toluene at 0 – 20℃; for 1h; Temperature; Green chemistry; Experimental Procedure 1.C; 2.C Target product synthesis Example 1: E8, E10-dodecadien-1-ol In a 1500L dry clean enamel reactor, enter 400kg of metered toluene, replace with nitrogen, put 46kg of chopped clean metal sodium, heat up to 80 ° C, cool under stirring, cool with ice water, cool to 10 ° C, drop by A mixture of intermediate B137 kg and intermediate C208.5 kg.Control 0-10 ° C drop, add dropwise, warm to 60 ° C, heat preservation reaction for 12 hours,Cool to 0 ° C, add 300 kg of water, 98 kg of sulfuric acid, control hydrolysis temperature 20 ° C, hydrolysis and stirring for 1 hour, check the water layer pH 3-4, layering, 200 kg of organic layer washed once, separate the water layer, organic layer negative pressure Concentrated to the solvent without, the remaining liquid enters the rectification column rectification, collecting 10mmHg, 118-120 ° C fraction, as shown in Figure 1,Detecting the E, E-isomer content of 99.5%,The quantity is: 141kg. The yield was 77.47%.The total yield was: 68%. | 77.47% |
Safety and Hazards
| Pictogram(s) |    |
| Signal | Danger |
| GHS Hazard Statements | H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H317 (64.62%): May cause an allergic skin reaction [Warning Sensitization, Skin] H334 (29.23%): May cause allergy or asthma symptoms or breathing difficulties if inhaled [Danger Sensitization, respiratory] H400 (99.23%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410 (63.85%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P272, P273, P280, P285, P302+P352, P304+P341, P321, P332+P313, P333+P313, P342+P311, P362, P363, P391, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 8; Packaging Group: III; UN Number: 3082 |
| Under the room temperature and away from light | |
| Storage | Under the room temperature and away from light |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 182.306 |
| logP | 4.548 |
| HBA | 1 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 20.23 |
| Rotatable Bond (RotB) | 8 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| Quantitative Results | ||
| 1 of 10 | Biological material | cat SV-CISM-2 cell line |
| Assay Description | Increase in cGMP formation in SV-CISM-2 cells relative to control upon treatment with the compound at 10e-4M | |
| Results | Activity not calculated | |
| Measurement | Activity | |
| 2 of 10 | Target | Multidrug resistance protein 1:Wild |
| Substance action on target | Inhibitor | |
| Assay Description | Inhibitory activity against P-glycoprotein mediated compound efflux was determined | |
| 3 of 10 | Biological material | cat SV-CISM-2 cell line |
| Assay Description | Formation of cGMP in SV-CISM-2 cells upon treatment with the compound at 10e-4M | |
| Results | Activity not calculated | |
| Measurement | Activity | |
| 4 of 10 | Biological material | cat SV-CISM-2 cell line |
| Assay Description | Formation of cGMP in SV-CISM-2 cells upon treatment with the compound at 10e-6M | |
| Results | Activity not calculated | |
| Measurement | Activity | |
| 5 of 10 | Biological material | cat SV-CISM-2 cell line |
| Assay Description | Increase in cGMP formation in SV-CISM-2 cells relative to control upon treatment with the compound at 10e-8M | |
| Results | Activity not calculated | |
| Measurement | Activity | |
| 6 of 10 | Biological material | cat SV-CISM-2 cell line |
| Assay Description | Increase in cGMP formation in SV-CISM-2 cells relative to control upon treatment with the compound at 10e-6M | |
| Results | Activity not calculated | |
| Measurement | Activity | |
| 7 of 10 | Biological material | cat SV-CISM-2 cell line |
| Assay Description | Formation of cGMP in SV-CISM-2 cells upon treatment with the compound at 10e-8M | |
| Results | Activity not calculated | |
| Measurement | Activity | |
| 8 of 10 | Effect | Behavioural Symptoms |
| Assay Description | Target : Ascogaster quadridentata Bioassay : bioassays conducted between hrs 2 and 12 of insects’ photophase (16:8 h light:dark cycle); parasitoids reaching filter paper contain. title comp. within 15 min classed as responders in vivo; vertical Y-shaped Pyrex glass olfactometers; 25-27 deg C; relat. humid. 50-70 percent; fluorescent “daylight”+wide-spectrum grow light; air drawn through apparatus; responses of walking of flying insects to odor sources record. | |
| 9 of 10 | Biological material | Cydia pomonella |
| Assay Description | Effect : pheromone activity Bioassay : dispensers: a piece of cotton dental roll (0.3-300 ng) and gray elastomer septa (0.1-30.0 μg); effect: upwind flights culminating in contact with the dispenser flight tunnel bioassay; males for bioassays were used three days after eclosion and 0.25-2.5 hr after darkening the room; flight tunnel: 210 cm in length, air speed 12 cm/sec, 23 deg C, dimly lighted overhead by red lights controlled by a rheostat | |
| Results | response: gray elastomer septa: ca. 10 to ca. 85 percent, cotton dental roll: 0 to ca. 70 percent | |
| 10 of 10 | Biological material | Cydia pomonella |
| Assay Description | Effect : pheromone activity Bioassay : effect: the number of males making a circling motion while wing fanning wing-flutter bioassay; pint fruit jar; males for bioassays were used 3 days after eclosion and 0.25-2.5 hr after darkening the room; the test room was dimly lighted by red lights; 3-mm-diameter glass rod with title comp. was inserted into the jar | |
| Results | response: 20.5-96.5 percent |
| Use Pattern |
| General chemicals |
| guest material for slow-release pheromon composition comprising mesoporous molecular sieve as host material |
| Chemical processes/laboratory use |
| synthesizing a sex pheromone of the codling moth |
| Agricultural use |
| method of controlling chestnut tortrix (Cydia splendana) |
| composition for controlling undesired insect infestation |
| Insect attractant |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watson Bio Ltd | https://www.watson-bio.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

![Structure of 4-[4-(acetyloxy)phenyl]-2-butanone CAS 3572-06-3](https://www.chemwhat.com/wp-content/uploads/2017/11/Structure-of-4-4-acetyloxyphenyl-2-butanone-CAS-3572-06-3-150x89.png)