Acetic acid CAS#: 64-19-7; ChemWhat Code: 881379
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN115626918 | Phenylfuran-tetrahydroisoquinoline compound as well as preparation and application thereof | 2023 |
Physical Data
| Appearance | Transparent ilquid,no visible impurities |
| Moisture | ≤0.115%(m/m) |
| Acetaldehyde | ≤0.030%(m/m) |
| Permanganate Time | ≥30min |
| Melting Point, °C |
| 16.7 |
| 16.6 |
| 16.53 |
| 16.6 |
| 16.5 – 16.55 |
| 16.5 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 117.94 | 760.051 |
| 117.79 | 760.014 |
| 117.9 | |
| 118.28 | 760.051 |
| 118 | |
| 117.9 | 760.051 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.049 | 19.99 |
| 1.02161 | 44.99 |
| 1.02704 | 39.99 |
| 1.03261 | 34.99 |
| 1.03844 | 29.99 |
| 1.0439 | 24.99 |
| 1.0469 | 24.99 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Adsorption isotherm | 25 | CF3 functionalized MIL-53(Al) | |
| Adsorption | C30H28N4O4 | ||
| Adsorption | SAPO-34 | ||
| Adsorption | neat (no solvent, gas phase) | aluminum oxide |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 13C | |||
| Chemical shifts, Spectrum | 1H | water-d2 | ||
| Chemical shifts | 1H | heavy water | 400 | |
| Chemical shifts | 1H | -163.16 | 500 | |
| Chemical shifts | 1H | heavy water | 400.1 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Spectrum | ||
| ATR (attenuated total reflectance), Bands, Spectrum | ||
| Spectrum | gas | -195.16 |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) |
| Spectrum | H2O | 175 – 350 nm |
| Spectrum | 190 – 240 nm | |
| Spectrum | 180 – 250 nm | |
| Absorption maxima |
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Acetic acid CAS 64-19-7
| Conditions | Yield |
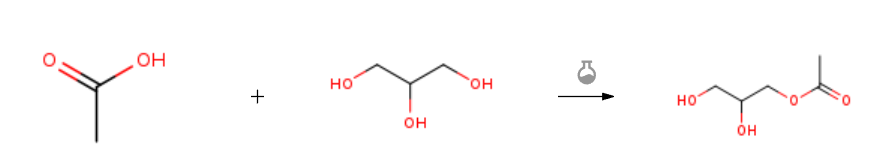
| With methanesulfonic acid In cyclohexane; butanone at 80℃; under 760.051 Torr; Reagent/catalyst; Temperature; Solvent; Large scale; Experimental Procedure Esterification raw material 9.2kgGlycerin and water-carrying agent 3kg butanone were added to a 30L atmospheric pressure reaction kettle, and the esterification reaction was carried out.It is a reversible reaction and produces water. In order to increase the conversion rate, water can be used to separate water from the reaction system. The material that can be used as a water-carrying agent must react with water to produce an azeotrope so that the water is more easily distilled out, and the solubility in water is small, and in this scheme, cyclohexane is used as a water-carrying agent.In addition, methanesulfonic acid was added to the reaction vessel as a catalyst for the esterification reaction, and the reaction vessel was heated to 80 ° C as a reaction temperature, and then 6 kg of another esterification raw material acetic acid was gradually added to the reaction vessel to cause it to occur. The esterification reaction produces glycerol monoacetate and water. Moreover, the reaction can be simultaneously applied to a reflux system with a water separator, and the water phase of the lower layer is separated by a water separator to obtain an oil phase of the upper layer, wherein the target product glycerin monoacetate is contained in the oil phase.The product in the reaction system can also be subjected to purification treatment. Specifically, the obtained oil phase is separated by a rectification column, and the water-carrying cyclohexane and the unreacted esterified raw material acetic acid in the oil phase are removed by vacuum distillation, and the heavy fraction at the bottom of the bottom is obtained. Pure glycerol monoacetate, the corresponding yield of this product is as high as 89.2%. | 89.2% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H226 (99.72%): Flammable liquid and vapor [Warning Flammable liquids] H314 (99.96%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] H318 (14.58%): Causes serious eye damage [Danger Serious eye damage/eye irritation] |
| Precautionary Statement Codes | P210, P233, P240, P241, P242, P243, P260, P264, P264+P265, P280, P301+P330+P331, P302+P361+P354, P303+P361+P353, P304+P340, P305+P354+P338, P316, P317, P321, P363, P370+P378, P403+P235, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
No data available
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 60.0526 |
| logP | -0.08 |
| HBA | 2 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 37.3 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Quantitative Results | |||
| 1 of 4,093 | Comment (Pharmacological Data) | Bioactivities present | |
| Reference | Derivatives of 2-(iminomethyl)amino-phenyl, their preparation, their use as medicaments and the pharmaceutical compositions containing them | ||
| 2 of 4,093 | Comment (Pharmacological Data) | Bioactivities present | |
| Reference | Process for purifying carbon dioxide-containing gas streams | ||
| 3 of 4,093 | Comment (Pharmacological Data) | Bioactivities present | |
| Reference | Substituted hydrazine mitomycin analogs | ||
| 4 of 4,093 | Comment (Pharmacological Data) | Bioactivities present | |
| Reference | Vitamin D3 metabolites. I. Synthesis of 25-hydroxycholesterol. | ||
| 5 of 4,093 | Comment (Pharmacological Data) | Bioactivities present | |
| Reference | Cob(I)alamin as catalyst. IV. Reduction of α,β-unsaturated nitriles | ||
| 6 of 4,093 | Comment (Pharmacological Data) | Bioactivities present | |
| Reference | Preparation of 29-acetoxy-30-norlupan-20-one derivatives with the substituted ring C | ||
| 7 of 4,093 | Comment (Pharmacological Data) | Bioactivities present | |
| Reference | STRUCTURE OF HELICOBASIDIN, A NOVEL BENZOQUINONE FROM HELICOBASIDIUM | ||
| 8 of 4,093 | Comment (Pharmacological Data) | Bioactivities present | |
| Reference | Bile acids and steroids. XXXV. Some A-ring aromatic steriods having oxygen functions at C-16 and their pharmacological activities. | ||
| 9 of 4,093 | Comment (Pharmacological Data) | Bioactivities present | |
| Reference | Studies on telomers and oligomers of vinylene carbonate. VI. Stereoselective conversion of vinylene carbonate telomers to trans unsaturated phosphate esters and their chemical behaviors | ||
| 10 of 10 | Comment (Pharmacological Data) | Bioactivities present | |
| Reference | Substituted cyclopropyl benzamides and pharmaceutical preparations and methods of use employing such compounds |
| Use Pattern |
| ACETIC ACID is manufacture of vinyl acetate, acetic anhydride, acetic ester, acetate, ethyl cellulose, and chloro acetic acid. It can also be used in the field of synthetic fiber, binding agent, pharmacy, fertilizer and dyeing raw material, and in the field of plastic, rubber and printing as solvent. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |