Actinomycin D CAS#: 50-76-0; ChemWhat Code: 97997
Identification
| Product Name | Actinomycin D |
| IUPAC Name | 2-amino-4,6-dimethyl-3-oxo-1-N,9-N-bis[(3R,6S,7R,10S,16S)-7,11,14-trimethyl-2,5,9,12,15-pentaoxo-3,10-di(propan-2-yl)-8-oxa-1,4,11,14-tetrazabicyclo[14.3.0]nonadecan-6-yl]phenoxazine-1,9-dicarboxamide |
| Molecular Structure | 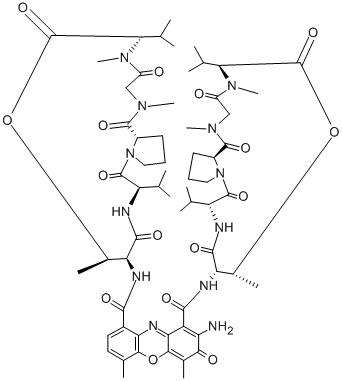 |
| CAS Registry Number | 50-76-0 |
| Synonyms | actinomycin D, Cosmegen, actinomycin C1, actinomycin IV, actinomycin X1, Actinomycin D, actinomycin-D |
| Molecular Formula | C62H86N12O16 |
| Molecular Weight | 1255.44 |
| InChI | InChI=1S/C64H88N10O16/c1-27(2)44-53(78)35-19-17-21-37(35)61(84)71(13)25-40(75)73(15)50(29(5)6)63(86)88-33(11)46(59(82)67-44)69-57(80)39-24-23-31(9)55-48(39)66-49-42(43(65)52(77)32(10)56(49)90-55)58(81)70-47-34(12)89-64(87)51(30(7)8)74(16)41(76)26-72(14)62(85)38-22-18-20-36(38)54(79)45(28(3)4)68-60(47)83/h23-24,27-30,33-38,44-47,50-51H,17-22,25-26,65H2,1-16H3,(H,67,82)(H,68,83)(H,69,80)(H,70,81) |
| InChI Key | RJURFGZVJUQBHK-IIXSONLDSA-N |
| Canonical SMILES | CC1C(C(=O)NC(C(=O)N2CCCC2C(=O)N(CC(=O)N(C(C(=O)O1)C(C)C)C)C)C(C)C)NC(=O)C3=C4C(=C(C=C3)C)OC5=C(C(=O)C(=C(C5=N4)C(=O)NC6C(OC(=O)C(N(C(=O)CN(C(=O)C7CCCN7C(=O)C(NC6=O)C(C)C)C)C)C(C)C)C)N)C |
| Isomeric SMILES | C[C@@H]1[C@@H](C(=O)N[C@@H](C(=O)N2CCC[C@H]2C(=O)N(CC(=O)N([C@H](C(=O)O1)C(C)C)C)C)C(C)C)NC(=O)C3=C4C(=C(C=C3)C)OC5=C(C(=O)C(=C(C5=N4)C(=O)N[C@H]6[C@H](OC(=O)[C@@H](N(C(=O)CN(C(=O)[C@@H]7CCCN7C(=O)[C@H](NC6=O)C(C)C)C)C)C(C)C)C)N)C |
USP Standard
| DEFINITION | Dactinomycin contains NLT 950 µg/mg and NMT 1030 µg/mg of dactinomycin (C62H86N12O16), calculated on the dried basis. [Caution—Great care should be taken to prevent inhaling particles of Dactinomycin and exposing the skin to it.] |
| IDENTIFICATION | |
| A. Ultraviolet Absorption | |
| Standard solution | 25 µg/mL of dactinomycin from USP Dactinomycin RS in methanol |
| Sample solution | 25 µg/mL in methanol |
| Analytical wavelengths | 240 and 445 nm |
| Acceptance criteria | |
| Absorptivity | The absorptivity, calculated on the dried basis,(ERR 1-Jun-2019) of the Sample solution at 445 nm is NLT 95.0% and NMT 103.0% that of the Standard solution. |
| Ratio | A240/A445, 1.30–1.50, Sample solution |
| B. | The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay. |
| ASSAY | |
| Procedure | |
| Mobile phase | Acetonitrile, 0.04 M sodium acetate, and 0.07 M acetic acid (46:25:25). Pass through a filter of 1-µm or finer pore size. The acetonitrile concentration may be varied to provide appropriate chromatographic system performance and a suitable elution time. |
| Standard solution | 1.2 mg/mL of USP Dactinomycin RS in Mobile phase. Use a freshly prepared solution, and protect it from light. |
| Sample solution | 1.2 mg/mL of Dactinomycin in Mobile phase. Use a freshly prepared solution, and protect it from light. |
| Chromatographic system | |
| Mode | LC |
| Detector | UV 254 nm |
| Column | 3.9-mm × 30-cm; packing L1 |
| Flow rate | 1 mL/min |
| Injection volume | 20 µL |
| System suitability | |
| Sample | Standard solution [Note—The retention time for dactinomycin is about 25 min.] |
| Suitability requirements | |
| Relative standard deviation | NMT 1.0%, dactinomycin (3 replicate injections) |
| Analysis | |
| Samples | Standard solution and Sample solution. Calculate the potency, in µg/mg, of dactinomyin (C62H86N12O16) in the Dactinomycin taken: Result = (Ru/Rs) × (Cs/Cu) × P × F Ru= peak response from the Sample solution; Rs= peak response from the Standard solution; Cs= concentration of USP Dactinomycin RS in the Standard solution (mg/mL); Cu= concentration of the Sample solution (mg/mL); P= potency of dactinomycin in USP Dactinomycin RS (mg/mg); F= conversion factor, 1000 µg/mg |
| Acceptance criteria | 950–1030 µg/mg on the dried basis |
| SPECIFIC TESTS | |
| Optical Rotation, Specific Rotation | |
| Sample solution | 1 mg/mL in methanol |
| Acceptance criteria | −293° to −329° (t = 20°) |
| Crystallinity | Meets the requirements |
| Loss on Drying | |
| Analysis | Dry under vacuum at a pressure not exceeding 5 mm of mercury at 60° for 3 h. |
| Acceptance criteria | NMT 5.0% |
| Bacterial Endotoxins Test | It contains NMT 100 USP Endotoxin Units/mg. |
| ADDITIONAL REQUIREMENTS | |
| Packaging and Storage | Preserve in tight containers, protected from light and excessive heat. |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US4826817 | Amino acid and hydroxyamino acid transporter compounds for therapeutic applications, process and use | 1989 |
| US4857553 | Method of treating nausea and vomiting with certain substituted-phenylalkylamino (and aminoacid) derivatives and other serotonin depleting agents | 1989 |
| US2003/87873 | Modified nucleosides for the treatment of viral infections and abnormal cellular proliferation | 2003 |
| US2009/53250 | Immune Modulating Oligonucleotides in Connection with Chemotherapeutic Measures | 2009 |
| US2010/111852 | Anti-Claudin 3 Monoclonal Antibody and Treatment and Diagnosis of Cancer Using the Same | 2010 |
| EP2275135 | THERAPEUTIC FOR HEPATIC CANCER | 2011 |
Physical Data
| Appearance | Bright red crystalline powder |
| Water Solubility | Slightly soluble |
| Melting Point, °C | Solvent (Melting Point) | Comment (Melting Point) |
| 238 – 240 | Decomposition | |
| 246 – 247 | ethanol, CHCl3 | |
| 246 – 247 |
| Solvent (Circular Dichroism) | Comment (Circular Dichroism) |
| various solvent(s) | 370 – 450 nm |
| 240 – 600 nm |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C |
| Chemical shifts | 1H | D2O | 24.9 |
| Chemical shifts | 1H | CDCl3 | |
| Chemical shifts | 13C | CDCl3 | |
| Chemical shifts | 1H | CDCl3, benzene-d6 |
| Description (Mass Spectrometry) |
| MALDI (Matrix assisted laser desorption ionization), time-of-flight mass spectra (TOFMS), spectrum |
| High resolution mass spectrometry (HRMS), tandem mass spectrometry, spectrum |
| Tandem mass spectrometry, CID (collision-induced dissociation), fragmentation pattern, spectrum |
| MALDI (Matrix assisted laser desorption ionization), time-of-flight mass spectra (TOFMS), fragmentation pattern, spectrum |
| Electrospray ionisation (ESI), spectrum |
| TOFMS (Time of flight mass spectrum), Tandem mass spectrometry, CID (collision-induced dissociation), ESI (Electrospray ionisation), LCMS (Liquid chromatography mass spectrometry), Spectrum |
| Spectrum, tandem mass spectrometry, collisionally activated dissociation (CAD) |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | dimethylsulfoxide | |||
| Spectrum | H2O, various solvent(s) | 350 – 600 nm | ||
| Absorption maxima | 443, 428, 240 | |||
| Absorption maxima | various solvent(s) | 424 | 21000 | |
| Absorption maxima | CHCl3 | 424, 442 | 21000, 23000 |
| Description (Adsorption (MCS)) | Partner (Adsorption (MCS)) |
| Further physical properties of the adsorbed molecule | supercoiled DNA |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Association with compound | H2O | 24.85 | DNR |
| NMR spectrum of the complex | D2O | 2 | /PRSGH562–502/ |
| NMR spectrum of the complex | D2O | d2 | |
| NMR spectrum of the complex | D2O | 2 | /PRSGH562–508/ |
| Further physical properties of the complex | 22 | d(GAAGCTTC)2 | |
| Further physical properties of the complex | supercoiled DNA | ||
| Stability constant of the complex with … | calf-thymus DNA |
Route of Synthesis

| Conditions | Yield |
| (i) H2, Pd-C, MeOH, (ii) aq. K3, DMF Multistep reaction | |
| Multi-step reaction with 2 steps 1: 94 percent / trifluoromethane-sulfonic acid / CH2Cl2 / 0.17 h / 23 °C 2: 1.) H2; 2.) potassium ferricyanide / 1.) 10percent palladium/charcoal / 1.) methanol, ethylacetate, 3 h; 2.) methanol, 23 deg C, 10 min |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H300: Fatal if swallowed [Danger Acute toxicity, oral] H350: May cause cancer [Danger Carcinogenicity] H360: May damage fertility or the unborn child [Danger Reproductive toxicity] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P201, P202, P264, P270, P281, P301+P310, P308+P313, P321, P330, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | UN number: 3462 Class: 6.1 Packing group: II |
| Under room temperature | |
| HS Code | 294200 |
| Storage | Store at 2~8°for long time, sealed and away from light. |
| Shelf Life | 2 years |
| Market Price | USD 10000/g |
| Use Pattern |
| Actinomycin D CAS#: 50-76-0 is used Pharmaceuticals |
| Actinomycin D CAS#: 50-76-0 is used for actinic chelitis |
| Actinomycin D CAS#: 50-76-0 is used for adenocarcinoma |
| Actinomycin D CAS#: 50-76-0 is used for basal cell carcinoma |
| benign tumors |
| carcinoma in situ |
| dysplastic oral cell carcinoma |
| epidermodysplasia verruciformis |
| erythroleukoplakia |
| hyperplastic skin lesion |
| oral squamous cell carcinoma |
| squamous cell carcinoma |
| cancer chemotherapeutic agent |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| ZHE JIANG HISUN PHARMACEUTICAL CO.,LTD | http://www.hisunpharm.com/en |
| SHANGHAI NEW ASIATIC PHARMACEUTICALS CO.,LTD. | http://www.xinyapharm.com/ |
| CAMING PHARMACEUTICAL LTD | https://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |
