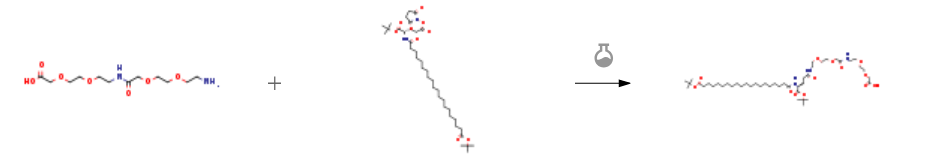
17-Amino-10-oxo-3,6,12,15-tetraoxa-9-azaheptadecanoic Acid CAS#: 1143516-05-5; ChemWhat Code: 1053913
Identification
Physical Data
| Appearance | Powder |
Spectra
No data available
Route of Synthesis (ROS)
| Conditions | Yield |
| With N-ethyl-N,N-diisopropylamine In ethanol at 20℃; Experimental Procedure To a solution of 2-(1 9-tert-l3utoxycarbonylnonade- canoylamino)pentanedioic acid 1 -tert-butyl ester 5-(2,5-di- oxopyrrolidin-1-yl) ester (2.50 g) and [2-(2-{2-[2-(2-amino- ethoxy)-ethoxy]acetylamino}ethoxy)ethoxy]acetic acid (alternative name: H-OEG-OEG-OH)(1 .47 g) in ethanol (40 mE) was added DIPEA (1.26 mE). The mixture was stirred at room temperature overnight and then concentrated in vacuo. To the residue was added aqueous 0.1 N HC1 (150 mE) and ethyl acetate (200 mE). The layers were separated and the aqueous layer was extracted with ethyl acetate (100 mE). The combined organic layers were washed with water and brine, dried (magnesium sulphate) and concentrated in vacuo to give an oil, which crystallised on standing. Yield 96% (3.1 g). ECMS: Theoretical mass: 874.2.Found: 874.49. | 96% |
| With N-ethyl-N,N-diisopropylamine In ethanol at 20℃; Experimental Procedure 16.1 Step 1: 19-{(S)-1-tert-Butoxycarbonyl-3-[2-(2-{[2-(2-carboxymethoxy-ethoxy)-ethylcarbamoyl]-methoxy}-ethoxy)-ethylcarbamoyl]-propylcarbamoyl}-nonadecanoic acid tert-butyl ester Step 1: 19-{(S)-1-tert-Butoxycarbonyl-3-[2-(2-{[2-(2-carboxymethoxy-ethoxy)-ethylcarbamoyl]-methoxy}-ethoxy)-ethylcarbamoyl]-propylcarbamoyl}-nonadecanoic acid tert-butyl ester (0556) (0557) To a solution of 2-(19-tert-Butoxycarbonylnonadecanoylamino)pentanedioic acid 1-tert-butyl ester 5-(2,5-dioxopyrrolidin-1-yl) ester (2.50 g, (prepared similarly as described in WO 2005/012347) and [2-(2-{2-[2-(2-Aminoethoxyl)ethoxy]acetylamino}ethoxy)ethoxy]acetic acid (1.47 g, alternative name: ∈-amino-3,6-dioxaoctanoic acid dimer, IRIS Biotech GmbH, Cat. No. PEG1221) in ethanol (40 ml) was added DIPEA (1.26 ml). The mixture was stirred at room temperature over night and then concentrated in vacuo. To the residue was added aqueous 0.1 N HCl (150 ml) and ethyl acetate (200 ml). The layers were separated and the aqueous layer was extracted with ethyl acetate (100 ml). The combined organic layers were washed with water and brine, dried (magnesium sulphate) and concentrated in vacuo to give an oil, which crystallized on standing. Yield 96% (3.1 g). LC-MS (electrospray): m/z=874.49. | 96% |
| With N-ethyl-N,N-diisopropylamine In ethanol at 20℃; | 96% |
Safety and Hazards
No data available
Other Data
| Transportation | Storage at 2~8°, Away from light. |
| Storage | Storage at 2~8°, Away from light. |
| Shelf Life | 1 year |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 308.332 |
| logP | -2.772 |
| HBA | 9 |
| HBD | 3 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 129.34 |
| Rotatable Bond (RotB) | 16 |
| Matching Veber Rules | 1 |
| Use Pattern |
| 17-Amino-10-oxo-3,6,12,15-tetraoxa-9-azaheptadecanoic Acid CAS#: 1143516-05-5 as an intermediate in the synthesis of semaglutide, developing an efficient synthesis route with this intermediate could contribute to the cost-effectiveness of producing semaglutide. |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |