Allopurinol CAS#: 315-30-0; ChemWhat Code: 1411684
Identification
| Product Name | Allopurinol |
| IUPAC Name | 1,5-dihydropyrazolo[3,4-d]pyrimidin-4-one |
| Molecular Structure | 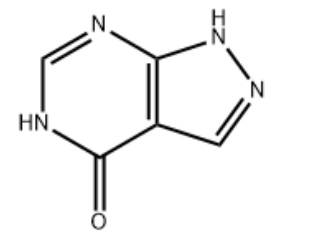 |
| CAS Registry Number | 315-30-0 |
| EINECS Number | 206-250-9 |
| MDL Number | MFCD00599413 |
| Synonyms | allopurinol 315-30-0 1H-Pyrazolo[3,4-d]pyrimidin-4-ol Zyloprim Lopurin Zyloric Suspendol Atisuril Bleminol Caplenal Takanarumin Uripurinol Embarin 4-Hydroxypyrazolo[3,4-d]pyrimidine 1H-Pyrazolo[3,4-d]pyrimidin-4(5H)-one Allopurinolum 1H-Pyrazolo(3,4-d)pyrimidin-4-ol 4-Hydroxypyrazolopyrimidine 4-Hydroxy-1H-pyrazolo(3,4-d)pyrimidine 1H-pyrazolo[3,4-d]pyrimidin-4(7H)-one 4-Hydroxy-3,4-pyrazolopyrimidine 4-Hydroxypyrazolo(3,4-d)pyrimidine Alopurinol [INN-Spanish] Allopurinolum [INN-Latin] 2H-Pyrazolo[3,4-d]pyrimidin-4-ol 1H-pyrazolo[3,4-d]pyrimidin-4(2H)-one 4-Hydroxypyrazolyl(3,4-d)pyrimidine 4H-Pyrazolo(3,4-d)pyrimidin-4-one 4′-Hydroxypyrazolol(3,4-d)pyrimidine 180749-09-1 1,5-Dihydro-4H-pyrazolo(3,4-d)pyrimidin-4-one 1,5-Dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one 1,5-Dihydro-4H-pyrazolo(3,4-d)pyrimidine-4-one 4H-Pyrazolo(3,4-d)pyrimidin-4-one, 1,5-dihydro- 4H-Pyrazolo[3,4-d]pyrimidin-4-one, 1,5-dihydro- 1,5-Dihydropyrazolo[3,4-d]pyrimidin-4-one MFCD00599413 HSDB 3004 EINECS 206-250-9 UNII-63CZ7GJN5I NSC-101655 63CZ7GJN5I SMR000059083 4-Hydroxy-1H-pyrazolo[3,4-d]pyrimidine ALLOPURINOL SODIUM ATH008 1H-Pyrazolo[3,4-d]pyrimidin-4-ol (9CI) 4H-Pyrazolo[3,4-d]pyrimidin-4-one, 1,7-dihydro- (9CI) 1H,2H,4H-pyrazolo[3,4-d]pyrimidin-4-one 1H,4H,7H-pyrazolo[3,4-d]pyrimidin-4-one DTXSID4022573 NSC1390 Allopurinol [USAN:INN:BAN:JAN] 4H-Pyrazolo[3,4-d]pyrimidin-4-one, 1,2-dihydro- NSC101655 9002-17-9 Allopurinol NCGC00015094-02 NCGC00094580-04 4H-Pyrazolo[3,4-d]pyrimidin-4-one, 2,5-dihydro- (9CI) 4H-Pyrazolo[3,4-d]pyrimidin-4-one, 2,7-dihydro- (9CI) Allohexal BW 56158 BW-56158 1H,4H,5H-pyrazolo[3,4-d]pyrimidin-4-one 1,5-Dihydro-pyrazolo[3,4-d]pyrimidin-4-one A 8003 DTXCID502573 4H-Pyrazolo[3, 1,5-dihydro- Jenapurinol Rimapurinol Allohexan Alloprin Allopurin Allorin Allpargin Capurate Novopurol Pureduct Tipuric Uribenz Uridocid WLN: T56 BMN GN INJ FQ Xanthomax Xanturic Roucol Zygout Apo-Allopurinol Xanthine oxidase 4-Hydroxypyrazolyl[3,4-d]pyrimidine Pan Quimica 4′-Hydroxypyrazolol[3,4-d]pyrimidine CAS-315-30-0 SR-05000001983 Hexanurat Uricto Quimica, Pan Allohexal (TN) 4-Hydroxypyrazol[3,4-D]pyrimidine Prestwick_511 Xanthomax-100 Xanthomax-300 4H-Pyrazolo[3,4-d]pyrimidin-4-one,1,2-dihydro- Zyloric-300 Allopurinol (Zyloprim) Spectrum_000026 ALLOPURINOL [MI] Opera_ID_1680 Lopac-A-8003 1,4-d]pyrimidin-4-one SCHEMBL4627 ALLOPURINOL [MART.] CHEMBL1467 NCIOpen2_001825 Lopac0_000102 ALLOPURINOL [USP-RS] ALLOPURINOL [WHO-DD] ALLOPURINOL [WHO-IP] BSPBio_001798 KBioGR_000550 KBioSS_000386 MLS001148183 US9138393, Allopurinol US9144538, Allopurinol DivK1c_000685 SPECTRUM1500108 SPBio_000056 ALLOPURINOLUM [WHO-IP] GTPL6795 SCHEMBL1128219 Allopurinol (JP17/USP/INN) BDBM35440 HMS502C07 KBio1_000685 KBio2_000386 KBio2_002954 KBio2_005522 KBio3_001298 ALLOPURINOL [EP IMPURITY] ALLOPURINOL [ORANGE BOOK] NINDS_000685 ALLOPURINOL [EP MONOGRAPH] BDBM181133 HMS1920A15 Pharmakon1600-01500108 ALLOPURINOL [USP MONOGRAPH] Allopurinol [USAN:BAN:INN:JAN] AMY18272 BCP26973 DRG-0056 DUZALLO COMPONENT ALLOPURINOL H-Pyrazolo(3,4-d)pyrimidin-4-ol HY-B0219 STR05189 Tox21_110082 4-Hydroxy-pyrazolo[3,4-d]pyrimidin AC-019 BBL009959 BDBM50016784 BDBM50140241 CCG-38916 NSC755858 s1630 SC1118 SC2251 STK378584 STK711106 AKOS000267490 Tox21_110082_1 Allopurinol, xanthine oxidase inhibitor TS-00028 SBI-0050090.P004 2H-Pyrazolo[3,4-d]pyrimidin-4-ol (9CI) A0907 EU-0100102 SW199406-4 1,5-dihydropyrazolo[3,4-d]-pyrimidin-4-one EN300-34144 VU0611037-1 BIM-0061756.0001 4h-pyrazolo[3,4-d]pyrimidin-4-one,1,7-dihydro- AB-323/25048497 Allopurinol (4-Hydroxypyrazolo[3,4-d]pyrimidine) Q412486 SR-01000075595 1,5-Dihydro-4H-pyrazolo-nu[3,4-d]pyrimidin-4-one 4H-pyrazolo[3,4-d]pyrimidin-4-one, 1,7-dihydro- J-504736 SR-01000075595-1 SR-05000001983-1 SR-05000001983-2 W-106892 4H-pirazolo [3,4-d] pirimidina-4-ona, 1,5-dihidro- F2173-0394 F3329-0375 Z104486670 Allopurinol, British Pharmacopoeia (BP) Reference Standard Allopurinol, European Pharmacopoeia (EP) Reference Standard Allopurinol, United States Pharmacopeia (USP) Reference Standard 1,5-Dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one Synonym: Allopurinol Allopurinol, Pharmaceutical Secondary Standard; Certified Reference Material 1H-Pyrazolo[3,4-d]pyrimidin-4-ol;1H-PYRAZOLO[3,4-D]PYRIMIDIN-4(5H)-ONE 184856-42-6 |
| Molecular Formula | C5H4N4O |
| Molecular Weight | 136.11 |
| InChI | InChI=1S/C5H4N4O/c10-5-3-1-8-9-4(3)6-2-7-5/h1-2H,(H2,6,7,8,9,10) |
| InChI Key | OFCNXPDARWKPPY-UHFFFAOYSA-N |
| Canonical SMILES | C1=NNC2=C1C(=O)NC=N2 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2001/53373 | Process and device for producing liquid dosage formulations of medicinal compounds on demand from tablets and capsules | 2001 |
Physical Data
| Appearance | Off-white powder |
| Melting Point, °C |
| 350 – 360 |
| 350 |
| 297 – 299 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Adsorption | H2O, HCl | 37 | cross-linked insoluble polyvinylpyrrolidone |
| Chemisorption | H2O | 37 | cross-linked insoluble polyvinylpyrrolidone |
| Chemisorption | H2O, HCl | 37 | cross-linked insoluble polyvinylpyrrolidone |
Spectra
| Description (IR Spectroscopy) |
| Spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | 210 – 330 nm | ||
| Spectrum | H2O | 200 – 280 nm | |
| Absorption maxima | H2O | 251 | |
| Spectrum | H2O | 240 – 280 nm |
Route of Synthesis (ROS)
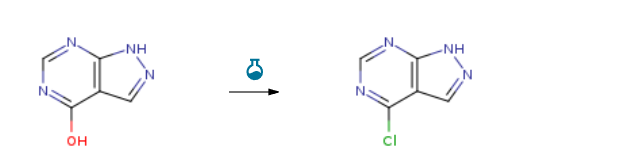
Route of Synthesis (ROS) of Allopurinol CAS#: 315-30-0
| Conditions | Yield |
| With N,N-dimethyl-formamide; trichlorophosphate for 12h; Reflux; | 76% |
| With trichlorophosphate In N,N-dimethyl-aniline for 1h; Reflux; | 72% |
| With trichlorophosphate In N,N-dimethyl-aniline at 80℃; for 2h; Experimental Procedure A mixture of allopurinol (2.00 g, 14.69 mmol) and N,N-dimethylaniline (2.00 g, 16.52 mmol) wasstirred in POCl3 (25 mL) at 80 °C for 2 h. The reaction mixture was diluted with water (35 mL), andextracted with ethyl acetate. The organic layer was washed with water and the organic phase wasconcentrated to dryness, and the residue was purified by column chromatography on silica gel using4:1.5 petroleum ether/ethyl acetate as eluent to give 2; white flake solid, 70 % yield. | 70% |
Safety and Hazards
No data available
Other Data
| Transportation | Under room temperature away from light |
| HS Code | |
| Storage | Under room temperature away from light |
| Shelf Life | 5 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 136.113 |
| logP | 0.905 |
| HBA | 4 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 74.69 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Quantitative Results | ||
| 1 of 800 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | Compositions suitable for the treatment of damage caused by ischemia/reperfusion or oxidative stress | |
| 2 of 800 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | FUSED BICYCLIC NATURAL COMPOUNDS AND THEIR USE AS INHIBITORS OF PARP AND PARP-MEDIATED INFLAMMATORY PROCESSES | |
| 3 of 800 | Comment (Pharmacological Data) | Bioactivities present |
| Reference | PROTEIN KINASE C ZETA INHIBITION TO TREAT VASCULAR PERMEABILITY |
| Use Pattern |
| Allopurinol CAS#: 315-30-0 is an intermediate in pesticides and dyes; pesticide raw materials; analytical reagents. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |