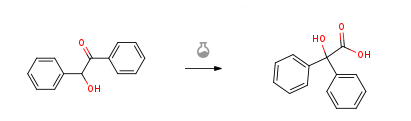
Benzilic acid CAS#: 76-93-7; ChemWhat Code: 27794
Identification
| Product Name | 3-Aminopyridine |
| IUPAC Name | 2-hydroxy-2,2-diphenylacetic acid |
| Molecular Structure | 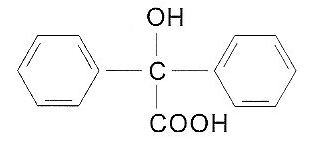 |
| CAS Registry Number | 76-93-7 |
| EINECS Number | 200-993-2 |
| MDL Number | MFCD00004447 |
| Beilstein Registry Number | No data available |
| Synonyms | Benzilic aciddiphenylglycolic acid2,2-diphenyl-2-hydroxyacetic acid2-hydroxy-2,2-diphenylacetic acidBZA |
| Molecular Formula | C14H12O3 |
| Molecular Weight | 228.24 |
| InChI | InChI=1S/C14H12O3/c15-13(16)14(17,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,17H,(H,15,16) |
| InChI Key | UKXSKSHDVLQNKG-UHFFFAOYSA-N |
| Canonical SMILES | O=C(O)C(O)(c1ccccc1)c1ccccc1 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN117185943 | Compound and preparation method thereof | 2023 |
| JP2022/74025 | COLORANT COMPOSITION, INK, RESIST COMPOSITION FOR COLOR FILTERS, HEAT-SENSITIVE TRANSFER RECORDING SHEET, TONER, AND WRITING INSTRUMENT | 2022 |
Physical Data
| Appearance | White crystalline powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) |
| 147 – 149 | tert-butyl methyl ether |
| 151.4 | ethanol |
| 143 – 145 | |
| 149 – 151 |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Stability constant of the complex with … | H2O | 20 | Th(4+) |
| Association with compound | |||
| Enthalpy of association |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | |
| Chemical shifts, Spectrum | 13C | dimethylsulfoxide-d6 | |
| Chemical shifts | 1H | chloroform-d1 | 500 |
| Chemical shifts | 13C | chloroform-d1 | 100 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands, Spectrum | potassium bromide | |
| Spectrum | nujol | |
| ATR (attenuated total reflectance), Bands | potassium bromide | |
| Spectrum | neat (no solvent, solid phase) |
| Description (Mass Spectrometry) |
| electron impact (EI), time-of-flight mass spectra (TOFMS), spectrum |
| high resolution mass spectrometry (HRMS), electrospray ionisation (ESI), spectrum |
| liquid chromatography mass spectrometry (LCMS), electron impact (EI), time-of-flight mass spectra (TOFMS), spectrum |
| HRMS (High resolution mass spectrometry), DART (direct analysis in real time), TOFMS (Time of flight mass spectrum), Spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Absorption maxima | methanol | 258, 230, 252, 264 | 492 | |
| Spectrum | aq. HCl | 240 – 280 nm | ||
| Spectrum | aq. H2SO4 | 240 – 280 nm | ||
| Absorption maxima | ethanol | 240 |
Route of Synthesis (ROS)
| Conditions | Yield |
| With potassium hydroxide In ethanol; water at 100℃; for 1h; Temperature; Sealed tube; | 70% |
| With potassium hydroxide | |
| With potassium hydroxide at 100℃; |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P264, P270, P301+P317, P330, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 228.247 |
| logP | -2.425 |
| HBA | 3 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 57.53 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Pharmaceutical intermediates Benzilic acid is mainly used as an intermediate in pharmaceutical synthesis, especially in the research and development and production of nonsteroidal anti-inflammatory drugs (NSAIDs) and antibacterial drugs. Its unique structure can be used as a starting material or key building block for the synthesis of drug molecules to generate compounds with analgesic, anti-inflammatory and other biological activities. |
| Organic synthesis reagents As a reagent for organic chemical reactions, benzilic acid can be used in esterification reactions, amidation reactions, and condensation reactions to synthesize more complex compounds. Due to the presence of its hydroxyl and carboxylic acid groups, the compound has high reactivity in various chemical syntheses and is suitable for the synthesis of heterocyclic compounds or coordination compounds. |
| Polymer and coating additives Benzilic acid can also be used as a polymer modifier or coating additive to improve the heat resistance, oxidation resistance and mechanical strength of materials. Its aromatic structure can enhance the thermal stability of polymers and maintain the stability of materials under high temperature conditions. |
| Metal complex synthesis Since the carboxylic acid group of benzilic acid can coordinate with metal ions to form stable metal complexes, it has potential applications in the development of catalysts and functional materials. These metal complexes can be used as catalysts to improve the efficiency of certain organic reactions. |
| Analytical Chemistry In analytical chemistry, benzilic acid can be used as a standard or reagent to detect and analyze the content and purity of certain compounds. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |