Benzotrithiophene CAS#: 29150-63-8; ChemWhat Code: 16079
Identification
| Product Name | Benzotrithiophene |
| IUPAC Name | 3,8,13-trithiatetracyclo[10.3.0.02,6.07,11]pentadeca-1(12),2(6),4,7(11),9,14-hexaene |
| Molecular Structure | 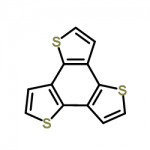 |
| CAS Registry Number | 29150-63-8 |
| EINECS Number | No data available |
| MDL Number | No data available |
| Beilstein Registry Number | No data available |
| Synonyms | benzo(1,2-b;3,4-b′;5,6-b′′)trithiophene, benzo[1,2-b:3,4-b′:5,6-b′′]trithiophene, benzo<1,2-b:3,4-b’:5,6-b”>trithiophene, benzo[1,2-b:3,4-b′:5,6-”]trithiophene, benzo[1,2-b:3,4-b’:5,6-b”]trithiophene, benzo-[1,2-b:3,4-b’:5,6-b”]trithiophene, benzo[1,2-b:3,4-b’:5,6-b”]trithiophene;CAS No:. 29150-63-8 |
| Molecular Formula | C12H6S3 |
| Molecular Weight | 246.371 |
| InChI | DKSAJSCNXULKER-UHFFFAOYSA-N |
| InChI Key | InChI=1S/C12H6S3/c1-4-13-10-7(1)11-9(2-5-14-11)12-8(10)3-6-15-12/h1-6H |
| Canonical SMILES | c1csc2c1c3c(ccs3)c4c2ccs4 |
| Patent Information |
| No data available |
Physical Data
| Appearance | White powder |
| Boiling point | 452.5±25.0 °C |
| Melting Point, °C |
| 452.5±25.0 °C |
| Boiling Point, °C |
| 251 |
| 250 – 252 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.53 | 19.85 |
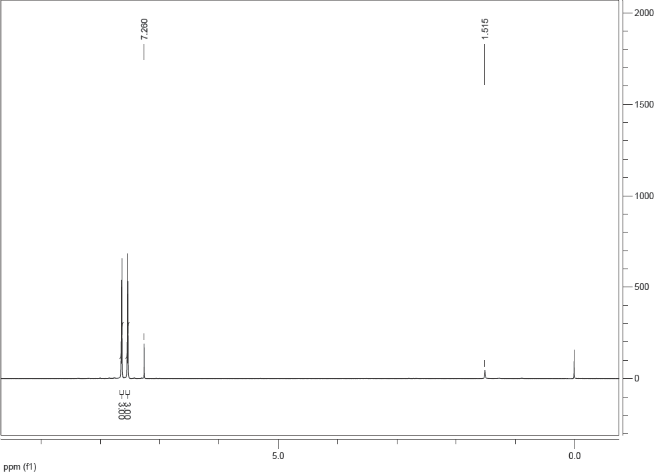
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | 400 | |
| Chemical shifts | 13C | chloroform-d1 | 101 | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 24.84 | 400.1 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 24.84 | 100.6 |
| DEPT (Distorsionless Enhancement by Polarisation Transfer), Chemical shifts, Spectrum | 13C | chloroform-d1 | 75 | |
| Spin-spin coupling constants | CDCl3 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Intensity of IR bands, Bands, Spectrum |
| Description (Mass Spectrometry) | Comment (Mass Spectrometry) | Peak |
| high resolution mass spectrometry (HRMS), electrospray ionisation (ESI), time-of-flight mass spectra (TOFMS), spectrum | ||
| HRMS (High resolution mass spectrometry), APCI (atmospheric pressure chemical ionization), Spectrum | ||
| EI (Electron impact) | Molecular peak | 246 m/z |
| EI (Electron impact) | mol peak | |
| spectrum, electron impact (EI) | ||
| spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | dichloromethane | 260, 288 | |
| methanol | 286, 262 | ||
| CH2Cl2 | 288, 267 | 16218, 69183 | |
| Absorption maxima | methanol | 263, 285 | 70600.1, 15000 |
| UV/VIS |
Route of Synthesis (ROS)
| Conditions | Yield |
| With N-Bromosuccinimide; acetic acid In chloroform at 60℃; Inert atmosphere; | 81% |
| With N-Bromosuccinimide; acetic acid In chloroform at 60℃; | 81% |
| With N-Bromosuccinimide In dichloromethane; acetic acid at 20℃; for 60h; Inert atmosphere; | 77% |
| With N-Bromosuccinimide In dichloromethane; acetic acid at 20℃; for 48h; Inert atmosphere; Darkness; Experimental Procedure 4 synthesis of 2,5,8-tribromobenzo[1,2-b:3,4-b’:5,6-b’]trithiophene Example 4 Synthesis of 2,5,8-Tribromobenzo[1,2-b:3,4-b’:5,6-b”]trithiophene (33) [Show Image] Under a nitrogen atmosphere, in a 50 mL three-neck flask, benzo[1,2-b:4,5-b’:5.6-b”]trithiophene (800 mg, 3.25 mmol) obtained in Synthesis Example 3, methylene chloride (22.7 mL) and acetic acid (5.7 mL) were added. Under light exclusion conditions, NBS (1.73 g, 9.74 mmol) was added little by little and stirred at room temperature 48 hours. After completion of the reaction, water (20 mL) was added. A precipitated solid substance was obtained by filtration and washed with ethanol (50 mL) and THF (20 mL) to obtain a light purple solid substance (1.2 g, 76%). 1H-NMR(270MHz,CDCl3)δ7.51(s,3H);M.S.(70eV,EI)m/z=484(M+) | 76% |
Safety and Hazards
| No data available |
| For more detailed information, please visit ECHA C&L website |
| Source: European Chemicals Agency (ECHA) License Note: Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: “Source: European Chemicals Agency, http://echa.europa.eu/”. Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. License URL: https://echa.europa.eu/web/guest/legal-notice Record Name: (1-Cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate URL: https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/213446 Description: The information provided here is aggregated from the “Notified classification and labelling” from ECHA’s C&L Inventory. Read more: https://echa.europa.eu/information-on-chemicals/cl-inventory-database |
Other Data
| Transportation | No data available |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Store at 2-8℃ for long time |
| Shelf Life | 2 years |
| Market Price | USD 1600/g |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 246.378 |
| logP | 4.347 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 84.72 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Used as the intermediates of the OLED |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |