BETA-PSEUDOURIDINE CAS#: 1445-07-4; ChemWhat Code: 109938
Identification
| Product Name | BETA-PSEUDOURIDINE |
| IUPAC Name | 5-[(2S,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1H-pyrimidine-2,4-dione |
| Molecular Structure | 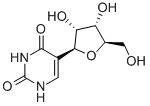 |
| CAS Registry Number | 1445-07-4 |
| MDL Number | MFCD00016582 |
| Synonyms | pseudouridine, 5-(β-D-ribofuranosyl)pyrimidine-2,4(1H,3H)-dione, 5-(β-D-ribofuranosyl)uracil, 5-β-D-ribofuranosyluracil, β-D-pseudouridine, β-pseudouridine, PURID, CAS Number 1445-07-4 |
| Molecular Formula | C9H12N2O6 |
| Molecular Weight | 244.204 |
| InChI | InChI=1S/C9H12N2O6/c12-2-4-5(13)6(14)7(17-4)3-1-10-9(16)11-8(3)15/h1,4-7,12-14H,2H2,(H2,10,11,15,16)/t4-,5-,6-,7+/m1/s1 |
| InChI Key | PTJWIQPHWPFNBW-GBNDHIKLSA-N |
| Canonical SMILES | C1=C(C(=O)NC(=O)N1)C2C(C(C(O2)CO)O)O |
| Isomeric SMILES | C1=C(C(=O)NC(=O)N1)[C@H]2[C@@H]([C@@H]([C@H](O2)CO)O)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2019/2906 | SYNTHESIS AND STRUCTURE OF HIGH POTENCY RNA THERAPEUTICS | 2019 |
| EP1142995 | ANTIURACIL MONOCLONAL ANTIBODY | 2001 |
| US5989911 | Site-specific synthesis of pseudouridine in RNA | 1999 |
| US5652358 | Solid-phase synthesis of oligoribonucleotides | 1997 |
| US2003/198980 | Heteroconfigurational polynucleotides and methods of use | 2003 |
| US2003/207266 | Asynchronous primed PCR | 2003 |
| US2005/3496 | Nucleic acid labeling compounds | 2005 |
| EP433452 | MONOCLONAL ANTIBODY, AND ASSAY METHOD, REAGENT KIT, SEARCH METHOD AND DRUG MISSILE USING SAID ANTIBODY | 1991 |
| US2003/7961 | Orthomolecular vitamin E derivatives | 2003 |
Physical Data
| Appearance | White to off-white powder |
| Melting Point, °C | Solvent (Melting Point) |
| 243 – 244 | methanol |
| 223 – 224 | ethanol |
| 219 – 220 | ethanol |
| 222 – 224 | methanol |
| Density, g·cm-3 | Type (Density) |
| 1.624 | crystallographic |
| Solvent (Circular Dichroism) | Comment (Circular Dichroism) |
| H2O | |
| various solvent(s) | 200 – 330 nm |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | dimethylsulfoxide-d6 | 26.94 | 500.01 |
| Chemical shifts, Spectrum | 1H | water-d2 | 26.84 | 500.01 |
| Chemical shifts, Spectrum | 13C | water-d2 | 26.84 | 125.8 |
| Chemical shifts, Spectrum | 13C | dimethylsulfoxide-d6 | 27.34 | 125.8 |
| Chemical shifts | 1H | tetradeuteriomethanol | 500 |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| aq. phosphate buffer | Remark: pH 7.4 | 263 | ||
| HCl | Remark: pH 2.18 | 263 | ||
| NaOH | Remark: pH 13.9 | 280 | ||
| Absorption maxima | aq. HCl | 263 | 7560 | |
| Absorption maxima | methanol | 264 | 4750 | |
| Absorption maxima | aq. NaOH | 285 | 7360 |
Route of Synthesis (ROS)
| Conditions | Yield |
| With hydrogenchloride In methanol at 20℃ for 5h | 93% |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Store at 2-8°C for long-term |
| Market Price | USD 300/g |
| Use Pattern |
| An isomer of the nucleoside uridine found in all species and in many classes of RNA except mRNA. It is formed by enzymes called synthases, which post-transcriptionally isomerize specific uridine res idues in RNA in a process termed pseudouridylation. Studies suggest that β-Pseudouridine reduces radiation-induced chromosome aberrations in human lymphocytes. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Ulcho Biochemical Ltd | https://www.ulcho.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

