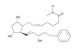
Bimatoprost CAS#: 155206-00-1; ChemWhat Code: 52735
Identification
| Product Name | Bimatoprost |
| IUPAC Name | (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(E,3S)-3-hydroxy-5-phenylpent-1-enyl]cyclopentyl]-N-ethylhept-5-enamide |
| Molecular Structure | 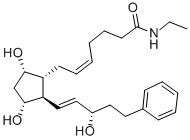 |
| CAS Registry Number | 155206-00-1 |
| Synonyms | bimatoprost, lumigan; (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentenyl]-5-N-ethylheptenamide, Bimatoprost, cyclopentane N-ethyl-heptenamide-5-cis-2-(3α-hydroxy-5-phenyl-1-trans-pentenyl)-3,5-dihydroxy, [1α,2β,3α,5α], (5Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenylpent-1-en-1-yl]cyclopentyl]-N-ethylhept-5-enamide, (5Z)-N-ethyl-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]-cyclopentyl]hept-5-enamide, (Z)-7-((1R,2R,3R,5S)-3,5-dihydroxy-2-((3S,E)-3-hydroxy-5-phenylpent-1-en-1-yl)cyclopentyl)-N-ethylhept-5-enamide |
| Molecular Formula | C25H37NO4 |
| Molecular Weight | 415.573 |
| InChI | InChI=1S/C25H37NO4/c1-2-26-25(30)13-9-4-3-8-12-21-22(24(29)18-23(21)28)17-16-20(27)15-14-19-10-6-5-7-11-19/h3,5-8,10-11,16-17,20-24,27-29H,2,4,9,12-15,18H2,1H3,(H,26,30)/b8-3-,17-16+/t20-,21+,22+,23-,24+/m0/s1 |
| InChI Key | AQOKCDNYWBIDND-FTOWTWDKSA-N |
| Canonical SMILES | CCNC(=O)CCCC=CCC1C(CC(C1C=CC(CCC2=CC=CC=C2)O)O)O |
| Isomeric SMILES | CCNC(=O)CCC/C=C\C[C@H]1[C@H](C[C@H]([C@@H]1/C=C/[C@H](CCC2=CC=CC=C2)O)O)O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| KR2017/25682 | Novel method for preparing Prostaglandin derivatives | 2017 |
| US2015/51410 | PROCESSES AND INTERMEDIATES FOR THE PREPARATIONS OF ISOMER FREE PROSTAGLANDINS | 2015 |
| US2015/158837 | Compound And Method | 2015 |
| US2015/31898 | PROCESS FOR PREPARATION OF PROSTAGLANDIN F2 ALPHA ANALOGUES | 2015 |
| US2014/135503 | NOVEL PROCESSES FOR THE PREPARATION OF PROSTAGLANDIN AMIDES | 2014 |
| WO2013/133730 | PROCESS FOR PREPARATION OF PROSTAGLANDIN F2α ANALOGUES | 2013 |
| WO2012/11128 | PREPARATION OF PROSTAGLANDIN DERIVATIVES | 2012 |
| WO2012/112451 | ESTER DERIVATIVES OF BIMATOPROST COMPOSITIONS AND METHODS | 2012 |
| WO2011/46569 | PROCESS FOR THE PREPARATION OF F-SERIES PROSTAGLANDINS | 2011 |
| WO2011/55377 | A NOVEL PROCESS FOR THE PREPARATION OF PROSTAGLANDINS AND INTERMEDIATES THEREOF | 2011 |
| US2007/286890 | Eyelash applicator and method | 2007 |
| US2005/69507 | Method for imparting artificial tan to human skin | 2005 |
| US2005/58614 | Methods for the treatment of gray hair using cyclopentane(ene) heptan(en)oic acid amides | 2005 |
Physical Data
| Appearance | White powder |
| Water Solubility | Slightly soluble(1.87e-02 g/L) |
| Melting Point, °C | Solvent (Melting Point) |
| 65.7 – 72.7 | ethyl acetate, tert-butyl methyl ether |
| 71.9 – 72.5 | water |
| 78 | |
| 75.9 | diethyl ether |
| 77.2 | methanol |
| 72.9 | water, ethanol |
| 62.1 | acetonitrile |
| log POW | Temperature (Partition octan-1-ol/water (MCS)), °C | pH |
| 3.41 | ||
| 2 | ||
| 2.4 | 25 | ~ 7.4 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz | Original Text (NMR Spectroscopy) |
| Chemical shifts | 1H | chloroform-d1 | 400 | 1H NMR (400 MHz; CDCl3) δH=1.09 (t, J=7.1 Hz, 3H, CH3), 1.42-2.40 (m, 14H, 6×CH2, 2×CH), 2.67 (m, 2H, CH2), 3.22 (dq, J=7.1, 6.3 Hz, 2H, CH2NH), 3.41 (broad s, 3H, 3×OH), 3.80-4.30 (broad m, 3H, 3×CHOH), 5.37 (m, 2H, 2×═CH), 5.47 (dd, J=15.2, 7.9 Hz, 1H, ═CH), 5.59 (dd, J=15.2, 7.9 Hz, 1H, ═CH), 5.90 (broad s, 1H, NH), 7.17 (m, 3H, ArCH’s), 7.26 (m, 2H, ArCH’s) |
| Chemical shifts | 1H | chloroform-d1 | 600 | 1H NMR (CDC13, 600 MHz, 25° C.) ö (ppm): 1.10 (t, J=7.2 Hz, 3H, —-CH2CH3), 1.46 (m, 1H, CH-i of cyclopentyl ring),1.62 (m, 1 H, one proton of CH2-3 group of a chain), 1.68 (m,H, one proton of CH2-3 group of a chain), 1.74 (m, 1 H, one proton of CH2-4 group of cyclopentyl ring), 1.78 (m, 1H, one proton of CH2-4 group of ca chain), 1.90 (m, 1H, one proton of CH2-4 group of w chain), 2.02-2.06(m, 2H, one proton of CH2-4 group and one of CH2-7 group of a chain), 2.11-2.15 (m, 3H, CH2-2 of a chain and one proton of CH2-4 group ofchain), 2.21 (m, 1H, one proton of CH2-4 group of cyclopentyl ring), 2.29 (m, 1H, one proton of CH2-7 group of a chain), 2.34 (m, 1H, CH-2cyclopentyl ring), 2.67 (m, 2H, CH2-S of w chain), 3.22 (m, 2H, -CCH3), 3.55 (s, 3H, three —-OH groups), 3.91 (m, 1H, CH-3 of cyclopentyl group), 4.08 (m, 1H, CH-3 of w chain), 4.12 (m, 1H, CH-S of cyclopentyl ring), 5.34 (m, 1H, CH-S of a chain), 5.41 (m, 1H, CH-6 of a chain), 5.47 (dd, J=9.0 and 15.3 Hz, 1H, CH-i ofw chain), S.S9 (dd, J=7.3 Hz and 1S.3 Hz, 1H, CH-2 ofw chain), S.98 (t, J=S.i Hz, 1H, >NH), 7.17 (m, 1H, H-4 aromatic), 7.18 (m, 2H, H-2 andH-6 aromatic), 7.26 (m, 2H, H-3 and H-S aromatic). |
| Chemical shifts | 13C | chloroform-d1 | 150 | 13C NMR (150 MHz, CDC13, 2S° C.) ö(ppm): 14.77 (——CH2CH3), 2S.38 (C-7 of a chain), 2S.63 (C-3 of a chain), 26.70 (C-4 of a chain), 31.88 (C-S of w chain), 34.40 (-CH2CH3), 3S.82 (C-2 of a chain), 38.7S(C-4 of w chain), 42.93 (C-4 of cyclopentyl ring), 50.19 (C-i of cyclopentyl ring), 55.47 (C-2 of cyclopentyl ring), 72.25 (C-3 of w chain), 72.33 (C-5 of cyclopentyl ring), 77.67 (C-3 of cyclopentyl ring), i25.77 (C-4 aromatic), i28.35 (2C, C-3 andC-5 aromatic), i28.35 (2C, C-2 andC-6 aromatic), 142.0 (C-i aromatic), i29.i8 (C-6 of a chain), i29.66 (C-5 of a chain), 133.20 (C-i of w chain), 135.12 (C-2 of w chain), 173.42 (CrrrO). |
| Chemical shifts | 13C | chloroform-d1 | 100 | 13C NMR (100 MHz; CDCl3) δC=14.8 (CH3), 25.4 (CH2), 25.6 (CH2), 26.7 (CH2), 31.9 (CH2), 34.4 (CH2NH), 35.8 (CH2C═O), 38.8 (CH2), 42.9 (CH2), 50.2 (CH), 55.5 (CH), 72.3 (CHOH), 72.4 (CHOH), 77.7 (CHOH), 125.8 (ArCH), 128.4 (2×ArCH), 128.5 (2×ArCH), 129.1 (═CH), 129.7 (═CH), 133.7 (═CH), 135.1 (═CH), 142.0 (ArC), 173.4 (C═O) |
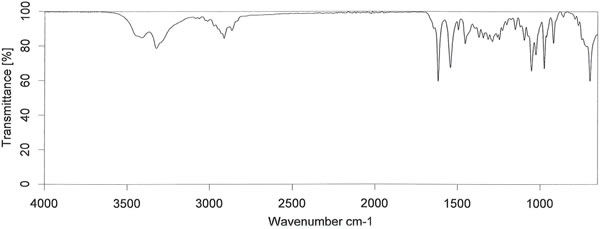
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Original Text (IR Spectroscopy) |
| Bands | potassium bromide | FT-IR (KBr) Vmax (cm’): 3420, 3327,3084, 3011,2914, 2865, 2933, 1620,1546,1496,1456,1372, 1317,1290,1249, 1151, 1097,1055,1027,976,920,698. |
| in KBr | IR: 3415.03cm-1, 3326.69cm-1, 3085.84cm-1, 2914.06cm-1, 2865.73cm-1, 1619.61cm-1, 1546.22cm-1, 1496.54cm-1, 1455.38cm-1, 1372.19cm-1, 1346.23cm-1, 1317.02cm-1, 1290.25cm-1, 1248.99cm-1, 1151.57cm-1, 1097.45cm-1, 1054.67cm-1, 1027.58cm-1, 975.78cm-1, 920.47cm-1, 859.10cm-1, 698.34cm-1 and 596.08cm-1 | |
| paraffin oil (nujol) | IR (Nujol): 3418.5, 3328.2, 3085.2, 3062.4, 2953.1, 2925.4, 2854.7, 1619.6, 1545.3, 1496.3, 1456.5, 1376.5, 1346.2, 1316.5, 1290.0, 1261.0, 1248.7, 1229.1, 1203.3, 1151.1, 1122.6, 1097.5, 1054.6, 1027.1, 975.9, 961.0, 920.3, 768.1, 721.8, 697.8, 595.7 and 545.4 cm-1 |
| Chromatographic data | Original string |
| TLC (Thin layer chromatography) | R&f%On a silica TLC (MTBE:EtOH 9:1 ): 0.35 |
| LC (Liquid chromatography) | Rt=22.56 mm. |
| HPLC (High performance liquid chromatography) | HPLC-MS (ESI): Kinetex XB-C 18, 2.7 μηι, kolumna 150 x 4.6 mm, (600 μ ΝΗ&3%· H&2%0 : 500 μ CH&3%COOH : 1 dm%3& H&2%0) : CH&3%CN (8:2, phase A)/ (600 μ ΝΗ&3% · H&2%0 : 500 μ CH&3%COOH : 1 dm%3& H&2%0) : CH&3%CN (8: 1, phase B) in concentration gradient 100percent – 75percent, 1.0 ml/min, R&t% = 21.79 min. (m/z = 416.3 [M + H]%+& for (15i?)-(+)-7b), R&t% = 22.33 min. (m/z = 416.3 [M + H]%+& for (5E, 15S)-(+)-7a), R&t% = 22.91 min. (m/z = 416.3 [M + H]%+& dla (155)- (+)-10a). |
| Type (Optical Rotatory Power) | Concentration (Optical Rotatory Power) | Enantiomeric excess, %ee | Solvent (Optical Rotatory Power) | Optical Rotatory Power, deg | Wavelength (Optical Rotatory Power), nm | Temperature (Optical Rotatory Power), °C |
| [alpha] | 1 g/100ml | methanol | 32.5 | 589 | 22 | |
| [alpha] | 1 g/100ml | chloroform | 39.07 | 589 | 20 | |
| [alpha] | 0.35 g/100ml | dichloromethane | 41.1 | 589 | 22 | |
| [alpha] | 1.0 weight percent | 98.72 | dichloromethane | 39.07 | 589 | 20 |
Route of Synthesis (ROS)
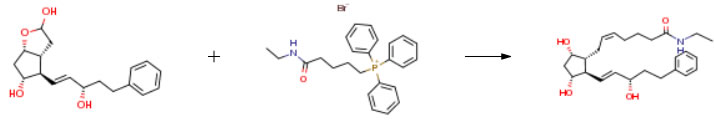
| Conditions | Yield |
| Stage #1: (3aR,4R,5R,6aS)-hexahydro-4-[(1E,3S)-3-hydroxy-5-phenyl-1-penten-1-yl]-2H-cyclopenta[b]furan-2,5-diol With potassium tert-butylate In tetrahydrofuran at 0℃; for 0.666667h; Stage #2: (5-(ethylamino)-5-oxopentyl)triphenylphosphonium bromide In tetrahydrofuran at 0℃; | 65% |
| Stage #1: (5-(ethylamino)-5-oxopentyl)triphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 0℃ for 0.666667h Inert atmosphere Stage #2: (3aR,4R,5R,6aS)-hexahydro-4-[(1E,3S)-3-hydroxy-5-phenyl-1-penten-1-yl]-2H-cyclopenta[b]furan-2,5-diol In tetrahydrofuran at 0 – 20℃ for 1h Inert atmosphere | 41% |
| Stage #1: (5-(ethylamino)-5-oxopentyl)triphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 0℃ for 1h Stage #2: (3aR,4R,5R,6aS)-hexahydro-4-[(1E,3S)-3-hydroxy-5-phenyl-1-penten-1-yl]-2H-cyclopenta[b]furan-2,5-diol In tetrahydrofuran at -17℃ for 20.5h Wittig Reaction | |
| Stage #1: (5-(ethylamino)-5-oxopentyl)triphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 0℃; for 0.666667h; Wittig Olefination; Schlenk technique; Inert atmosphere; Stage #2: (3aR,4R,5R,6aS)-hexahydro-4-[(1E,3S)-3-hydroxy-5-phenyl-1-penten-1-yl]-2H-cyclopenta[b]furan-2,5-diol In tetrahydrofuran at 20℃; for 1h; Wittig Olefination; Schlenk technique; Inert atmosphere; | 82.6mg |
| Stage #1: (5-(ethylamino)-5-oxopentyl)triphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 0℃; for 0.666667h; Inert atmosphere; Stage #2: (3aR,4R,5R,6aS)-hexahydro-4-[(1E,3S)-3-hydroxy-5-phenyl-1-penten-1-yl]-2H-cyclopenta[b]furan-2,5-diol In tetrahydrofuran at 0 – 20℃; for 2h; Inert atmosphere; | 108 mg |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H302: Harmful if swallowed [Warning Acute toxicity, oral] H312: Harmful in contact with skin [Warning Acute toxicity, dermal] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H340: May cause genetic defects [Danger Germ cell mutagenicity] H360: May damage fertility or the unborn child [Danger Reproductive toxicity] H361: Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P201, P202, P264, P270, P280, P281, P301+P312, P302+P352, P305+P351+P338, P308+P313, P312, P322, P330, P337+P313, P363, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under 2-8℃ for shipment | |
| HS Code | 293750 |
| Storage | Under -20℃ for long time storage |
| Shelf Life | 1 year |
| Market Price | USD 450/g |
| Use Pattern |
| Bimatoprost CAS#: 155206-00-1 can be used in pharmaceuticals |
| Bimatoprost CAS#: 155206-00-1 can be used for treating hair loss of the eyebrows |
| Bimatoprost CAS#: 155206-00-1 is used for treating hypotrichosis associated with chemotherapy treatment regimens |
| Bimatoprost CAS#: 155206-00-1 is used for treating hypotrichosis of the eyelashes |
| treatment of dry-eye and related symptoms |
| treating or preventing skin diseases or disorders |
| treating or preventing vitreous adhesions |
Related Chemicals
Buy Reagent | |
| Sigma-Aldrich P6740 | 1mg, 95% |
| ChemWhat 52735 | 1g, 5g, 10g, 50g, 99.5% |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | https://www.caming.com/bimatoprost-cas-155206-00-1/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |