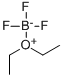Boron trifluoride tetrahydrofuran complex CAS#: 462-34-0; ChemWhat Code: 487522
Identification
| Product Name | Boron trifluoride tetrahydrofuran complex |
| IUPAC Name | trifluoro(oxolan-1-ium-1-yl)boranuide |
| Molecular Structure | 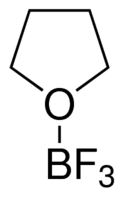 |
| CAS Registry Number | 462-34-0 |
| EINECS Number | 207-325-9 |
| MDL Number | MFCD00040372 |
| Synonyms | boron trifluoride-tetrahydrofuran complex, trifluoro-tetrahydrofuran-1-ium-1-yl-boron, boron trifluoride tetrahydrofuran complex, boron trifluoridetetrahydrofuran complex, borontrifluoride tetrahydrofuran complex, trifluoroborane-tetrahydrofuran complex, boron trifluoride tetrahydrofuran |
| Molecular Formula | BF3 · THF |
| Molecular Weight | 139.91 |
| InChI | InChI=1S/C4H8BF3O/c6-5(7,8)9-3-1-2-4-9/h1-4H2 |
| InChI Key | XYMZNNGTHKHCJH-UHFFFAOYSA-N |
| Canonical SMILES | [B-]([O+]1CCCC1)(F)(F)F |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2003/236430 | Preparation of protected amino acids | 2003 |
| EP1428827 | Derivatives of 2,2′-Di (3,4-alkylenedioxythiophen) their preparation and use | 2004 |
| WO2006/34459 | SUBSTITUTED ARYLPYRAZOLES | 2006 |
Physical Data
| Appearance | Yellowish or light yellow transparent liquid |
| Water Solubility | Slightly soluble(1.87e-02 g/L) |
| Melting Point | −123 °C(lit.) |
| Boiling Point | 180 °C(lit.) |
| Solubility | Miscible with dimethyl formamide. |
| Water solubility | hydrolysis |
| Density | 1.268 g/mL at 25 °C(lit.) |
| Sensitivity | Moisture Sensitive |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 11B | chloroform-d1 | 128.33 |
Route of Synthesis (ROS)
| Conditions | Yield |
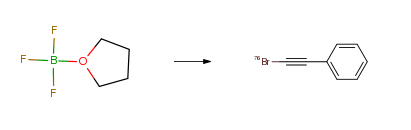
| With peracetic acid; ammonium 76Br-bromide; ammonia In methanol; water at 20℃; for 0.166667h; Experimental Procedure No-carrier-added NH476Br (3.7 MBq in 0.1percent aqueous NH4OH) was placed into a 2 mL Wheaton vial containing trifluoroborate (100 μL of 5.2.x.10-2 M solution in 50percent aqueous tetrahydrofuran). To this was added peracetic acid (100 μl, 0.3percent solution in methanol). The reaction vial was sealed, covered with aluminum foil, and the mixture stirred for 10 min at room temperature. The radiobrominated product was isolated by passing through a silica gel Sep-Pak cartridge using petroleum ether as eluent. The radiochemical purity of the 1-[76Br]bromo-2-phenylethyne was determined by radio-TLC (aluminum backed silica gel plate, hexane) Rf=0.73. The radiochemical (decay corrected) was found to be 80percent and the radiochemical purity was >98percent. The total synthesis time was 20 min. Alkenyltrifluoroborates were radiobrominated using the same procedure. | 80% |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | No data vailable |
| Market Price | No data vailable |
| Use Pattern |
| Boron trifluoride tetrahydrofuran complex CAS#: 462-34-0 as a acid additive as Lewis acid |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |