Ultraviolet absorber UV-326 CAS#:3896-11-5; ChemWhat Code: 50770
Identification
| Product Name | Ultraviolet absorber UV-326 |
| IUPAC Name | 2-tert-butyl-6-(5-chlorobenzotriazol-2-yl)-4-methylphenoll |
| Molecular Structure | 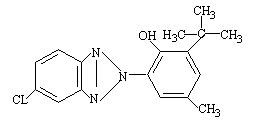 |
| CAS Registry Number | 3896-11-5 |
| EINECS Number | 223-445-4 |
| MDL Number | MFCD00059707 |
| Beilstein Registry Number | No data available |
| Synonyms | 2-(3-tert-butyl-2-hydroxy-5-methylphenyl)-2H-5-chlorobenzotriazole |
| Molecular Formula | C17H18ClN3O |
| Molecular Weight | 315.797 |
| InChI | InChI=1S/C17H18ClN3O/c1-10-7-12(17(2,3)4)16(22)15(8-10)21-19-13-6-5-11(18)9-14(13)20-21/h5-9,22H,1-4H3 |
| InChI Key | OCWYEMOEOGEQAN-UHFFFAOYSA-N |
| Canonical SMILES | Cc1cc(c(c(c1)n2nc3ccc(cc3n2)Cl)O)C(C)(C)C |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2020/325107 | BENZOTRIAZOLE COMPOUND | 2020 |
| US2019/107647 | OPTICAL MATERIAL COMPRISING A RED-SHIFTED BENZOTRIAZOLE UV ABSORBER | 2019 |
| CN108148009 | A transfer method using catalytic preparation of benzo triazole ultraviolet ray absorbing agent (by machine translation) | 2018 |
| WO2013/188490 | STABILIZER COMPOSITIONS CONTAINING SUBSTITUTED CHROMAN COMPOUNDS AND METHODS OF USE | 2013 |
Physical Data
| Appearance | Light yellow powder |
| Solubility | No data available |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Melting Point, °C | Solvent (Melting Point) |
| 140 – 141 | |
| 140 – 141 | ethanol, CH2Cl2 |
| 139 – 141 | |
| 141.5 – 142 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Comment (NMR Spectroscopy) |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | |
| Chemical shifts | 13C | chloroform-d1 | |
| Chemical shifts | 13C | neat (no solvent, solid phase) | |
| Chemical shifts | 15N | neat (no solvent, solid phase) | |
| Chemical shifts, Spectrum | 15N | chloroform-d1 | |
| HMBC (Heteronuclear Multiple Bond Coherence), Spectrum | 1H,15N | chloroform-d1 |
| Description (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | 3080 cm**(-1) |
| IR |
| Description (Mass Spectrometry) |
| liquid chromatography mass spectrometry (LCMS), tandem mass spectrometry, spectrum |
| spectrum |
| negative ion spectroscopy |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum | dichloromethane | |
| Spectrum | chloroform | 353 |
| Spectrum | chloroform | 353 |
| Spectrum | 353.5 |
Route of Synthesis (ROS)
| Conditions | Yield |
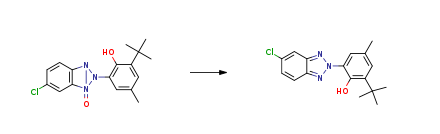
| With platinum on activated charcoal; hydrogen at 50 – 75℃; under 3750.38 – 6000.6 Torr; Autoclave; Experimental Procedure Step 3: The intermediate nitrogen oxide solution of formula II obtained in step 2 is transferred to an autoclave, Pt / C catalyst is introduced and hydrogen gas is introduced; the reaction temperature is controlled at 50-65 DEG C and the hydrogen pressure is adjusted to 0.5 to 0.8 MPa (constant), when the hydrogen absorption amount is lower than 0.05MPa / min, and the temperature is raised to 75 DEG C for 0.5 to 3 hours, the hydrogen reduction reaction is completed; Step 4: The mixed solution obtained in Step 3 is filtered to remove the catalyst,Reducing the temperature of the reducing liquid to 5-10 DEG C to precipitate the solid, separating the aqueous phase and adjusting the pH to 8.5-9.5,Precipitating a solid; combining the two solids to give a crude product of the trisazolyl type ultraviolet absorber of formula III;Step 5: A portion of the second solvent was added to the crude product obtained in Step 4,Heating to 50 to 75 DEG C and holding for 0.5 to 2 hours,Keeping and slowly adding the remaining second solvent to the system near the dissolution or just clear (visual),The filtrate was cooled to 0 to 10 ° C after hot filtration, and the precipitated solid was collected,To obtain a benzotriazole-based light stabilizer of high purity as shown in Formula III; | 92.1% |
Safety and Hazards
| GHS Hazard Statements | H413 (88.99%): May cause long lasting harmful effects to aquatic life [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P273, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | No data available |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 315.802 |
| logP | 6.455 |
| HBA | 3 |
| HBD | 1 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 50.94 |
| Rotatable Bond (RotB) | 2 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Ultraviolet absorber UV-326 CAS#:3896-11-5 Environmental protection |
| Ultraviolet absorber UV-326 CAS#:3896-11-5 UV absorber |
| Ultraviolet absorber UV-326 CAS#:3896-11-5 UVA (ultraviolet absorber) |
| Ultraviolet absorber UV-326 CAS#:3896-11-5 ultraviolet ray absorbing agent |
| UV absorber for dye liquor |
| precipitating additive for antimicrobial thermoplastic elastomer compositions |
| useful as UV absorbing and light stabilizing additive in plastic films and fibers |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |