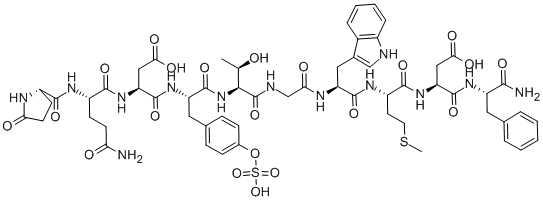
CAERULEIN CAS#: 17650-98-5; ChemWhat Code: 98454
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US4351829 | Use of polypeptides as analgesic drugs | 1982 |
Physical Data
| Appearance | White Powder |
Spectra
No data available
Route of Synthesis (ROS)
| Conditions | Yield |
Experimental Procedure XIII N-terminal tetrapeptide sulfate ester hydrazide of CRL butyl ester and coupled with DCC to the Tyr-sulfate ester-resin. After deblocking with 50% TFA/CH2 Cl2 (v/v), BOC-Glu(p-nitrophenyl ester) and pGlu(pentafluorophenyl ester) are respectively used for stepwise coupling. The tetrapeptide sulfate ester is cleaved from the resin in DMF with 30-fold excess hydrazine and isolated by precipitation with ethyl ether. It is further purified by precipitation from DMF with ethylacetate, yielding a pure Caerulein 1-4 tetrapeptide hydrazide suitable for further synthesis. |
Safety and Hazards
| GHS Hazard Statements | Not Classified |
Source: European Chemicals Agency (ECHA)
License Note: Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: “Source: European Chemicals Agency, http://echa.europa.eu/”. Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.
License URL: https://echa.europa.eu/web/guest/legal-notice
Record Name: (1-Cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate
URL: https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/213446
Description: The information provided here is aggregated from the “Notified classification and labelling” from ECHA’s C&L Inventory. Read more: https://echa.europa.eu/information-on-chemicals/cl-inventory-database
Other Data
| Transportation | Store at -20°C for long time, in container tightly sealed; Protect from light. |
| HS Code | |
| Storage | Store at -20°C for long time, in container tightly sealed; Protect from light. |
| Shelf Life | 2 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 1352.42 |
| logP | -5.328 |
| HBA | 33 |
| HBD | 17 |
| Matching Lipinski Rules | 1 |
| Veber rules component | |
| Polar Surface Area (PSA) | 585.08 |
| Rotatable Bond (RotB) | 47 |
| Matching Veber Rules | 0 |
| Use Pattern |
| Frogletin (also known as Frog Dermaseptin) is a type of antimicrobial peptide extracted from frog skin, with a variety of biological activities. Its main functions include: Antimicrobial Activity: Frogletin has significant inhibitory or killing effects on various pathogens, including bacteria, fungi, and viruses. This gives it potential applications in fighting infections, especially those caused by multidrug-resistant bacteria. Antitumor Activity: Some studies have shown that Frogletin peptides can selectively kill certain cancer cells without significantly harming normal cells, making it a promising candidate for cancer treatment. Immune Modulation: Frogletin has the potential to regulate immune responses by stimulating or suppressing immune cell activity, enhancing the body’s defense against infections and diseases. Anti-inflammatory Effects: In certain cases, Frogletin exhibits anti-inflammatory properties, helping to reduce inflammation caused by infections or immune reactions. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |