Cesium formate CAS#: 3495-36-1; ChemWhat Code: 27423
Identification
| Product Name | Cesium formate |
| IUPAC Name | cesium;formate |
| Molecular Structure | 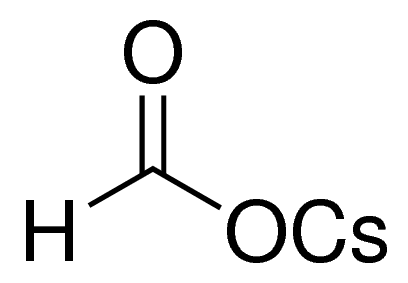 |
| CAS Registry Number | 3495-36-1 |
| EINECS Number | 222-492-8 |
| MDL Number | MFCD00039103 |
| Beilstein Registry Number | 3912426 |
| Synonyms | cesium formate, cesium(I) formate, caesium formate, formic acid ; cesium formate, Ameisensaeure; Caesiumformiat |
| Molecular Formula | CHO2*Cs |
| Molecular Weight | 177.923 |
| InChI | InChI=1S/CH2O2.Cs/c2-1-3;/h1H,(H,2,3);/q;+1/p-1 |
| InChI Key | ATZQZZAXOPPAAQ-UHFFFAOYSA-M |
| Canonical SMILES | C(=O)[O-].[Cs+] |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US3944588 | Catalytic process for polyhydric alcohols and derivatives | 1976 |
| US4151192 | Promoting n-propyl alcohol formation with vanadium compounds | 1979 |
| US5382736 | Method for catalytic dechlorination of polychlorinated biphenyls | 1995 |
| US5874474 | Topical application for relief of adverse skin condition for animals | 1999 |
| US2002/143209 | Methods of making cesium salts and other alkali metal salts | 2002 |
| EP819098 | PROCESS FOR THE PRODUCTION OF CESIUM COMPOUNDS | 2003 |
Physical Data
| Appearance | Crystals with lumps |
| Water Solubility | 846 g/l at 20 °C |
| Vapour Pressure | 4.80 hPa at 25 °C |
| Auto-ignition Temperature | < 42 °C (< 108 °F) at 1,013 hPa |
| Melting Point, °C |
| 173 |
| 261.85 – 263.85 |
| 263 |
| 266 |
| 265 |
| Description (Electrical Data) | Electric Conductivity, S/cm | Temperature of Electric Conductivity, °C |
| Electrical conductivity | 0.342 | 266 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Original Text (NMR Spectroscopy) |
| Chemical shifts | 13C | d(4)-methanol | 13C NMR (CD3OD, in ppm): 53 (-OMe), 158 (-CO-), 161 (CsHCOs), and 171 (CsHCOO). | |
| Linewidth of NMR absorption | 1H | solid | 46.85 – 256.85 | |
| Linewidth of NMR absorption | 133Cs | solid | 46.85 – 256.85 | |
| Linewidth of NMR absorption | 133Cs | formic acid | 25 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | neat (no solvent) | 2800 cm**(-1) |
| Spectrum | not given | 400 cm**-1 – 4000 cm**-1 |
Route of Synthesis (ROS)
| Conditions | Yield |
| Stage #1: carbon dioxide; carbon monoxide; caesium carbonate With silica gel; pyrographite at 325℃; under 15001.5 – 33753.4 Torr; Stage #2: methanol at 200℃; under 30003 Torr; for 2h; Reagent/catalyst; Temperature; Pressure; Experimental Procedure 3 (One-Step Process for the Preparation of Dimethyl Oxalate with C02, CO, and Cs2CO3 Cs2CO3/5i02 or A1203 and charcoal) Activated charcoal was vacuum dried overnight at 90 °C. The dried activated charcoal was mixed with equal amount of engineering silica (H-53), pre-calcined for two hours at 400 °C under inert conditions (e.g. glove box). The resultant charcoal/silica mixture wasused as a catalyst support for the reaction catalyst, cesium carbonate. Cesium carbonate (0.8 g) with equal amount of charcoal and silica (0.2 g each) was placed in a high pressure reactor (100 mL Parr (Parr Instrument Company, U.S.A.) reactor. C02 (45 bar, 4.5 MPa) was charged and the reactor heated to 325 °C, maintained at 325 °C and cooled to room temperature to form the adduct. CO (20 bar, 2 MPa) was then charged and the mixture was stirred for 1-2 hour at325 °C with agitation, and then cooled to room temperature by applying cool air to the reactor. The reactor was cooled to 25 °C and depressurized. The reaction mixture contained cesium oxalate, cesium formate, and cesium bicarbonate as confirmed by ‘3C NIVIR. A solution of methanol (20 mL) and the crude cesium oxalate was add to the reactor, and the reactor was pressurized with C02 (40 bar, 4.0 MPa). The mixture was heated for 2 hours at 200 °C, andthen cooled. The remaining solvent (methanol) was removed by evaporation under vacuum. The product composition was analyzed and identified as being a mixture of dimethyl oxalate, cesium formate, and cesium bicarbonate. The overall yield of DM0 was 95percent, respectively. the overall yield of cesium formate as byproduct was about 7-8percent. ‘3C NIVIR (CD3OD, in ppm):53 (-OMe), 158 (-CO-), 161 (C5HCO3), and 171 (CsHCOO). A mixture of alumina/activatedcharcoal with cesium carbonate gave similar results under the same conditions. | A 95% B n/a C 0.8% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Warning |
| GHS Hazard Statements | H302: Harmful if swallowed [Warning Acute toxicity, oral] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H371: May cause damage to organs [Warning Specific target organ toxicity, single exposure] H373: Causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P260, P264, P270, P280, P301+P312, P305+P351+P338, P309+P311, P314, P330, P337+P313, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 291512 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price |
| Use Pattern |
| Cesium formate CAS#: 3495-36-1 is used for treating drug addiction |
| molybdenum- and sulfur-containing catalyst impregnation to promote synthesis of an alcohol from syngas |
| Cesium formate CAS#: 3495-36-1 is a component of antifungal composition for controlling the growth of vegetative fungi from spores |
| Drilling & completion fluid |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Cabot Corporation | http://www.cabotcorp.com/ |
| Warshel Chemical Ltd | https://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |

