Chloroacetic acid sodium salt CAS#: 3926-62-3; ChemWhat Code: 37690
Identification
| Product Name | Chloroacetic acid sodium salt |
| IUPAC Name | sodium;2-chloroacetate |
| Molecular Structure | 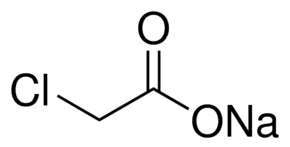 |
| CAS Registry Number | 3926-62-3 |
| EINECS Number | 223-498-3 |
| MDL Number | MFCD00002684 |
| Beilstein Registry Number | 3597157 |
| Synonyms | sodium monochloroacetic acid, monochloroacetic acid sodium salt, chloroacetic acid, sodium salt, chloroacetic acid sodium salt, sodium α-chloroacetate, sodium monochloroacetate, sodium 2-chloroacetate CAS No.: 3926-62-3 CAS Number: 3926-62-3 CAS#: 3926-62-3 |
| Molecular Formula | ClCH2COONa |
| Molecular Weight | 116.48 |
| InChI | InChI=1S/C2H3ClO2.Na/c3-1-2(4)5;/h1H2,(H,4,5);/q;+1/p-1 |
| InChI Key | FDRCDNZGSXJAFP-UHFFFAOYSA-M |
| Canonical SMILES | C(C(=O)[O-])Cl.[Na+] |
Physical Data
| Appearance | White crystalline powder |
| Solubility | 440 g/L (20 ºC) |
| Flash Point | 270°C |
| Sensitivity | Hygroscopic |
| Melting Point, °C | Solvent (Melting Point) |
| 199 °C |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz | Comment (NMR Spectroscopy) |
| Chemical shifts, Spectrum | 1H | water-d2 | 25 | 300 | |
| Chemical shifts | 1H | D2O | 200 | ||
| Chemical shifts | 13C | D20 | 50.31 | ||
| Spectrum | 13C | solid | 75.49 | ||
| Spectrum | 23Na | solid | 140 | 79.4 | time dependence |
| Spin-spin coupling constants | 1H-13C. Solvent(s): neat (no solvent, solid phase) | ||||
| Chemical shifts | 1H | D20 | |||
| Chemical shifts | 13C | solid | 19.9 | ||
| Spectrum | 13C | solid | 19.9 | ||
| NMR |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C | Comment (IR Spectroscopy) |
| Spectrum | potassium bromide | ||
| ATR (attenuated total reflectance), Bands | |||
| Spectrum | gas | ||
| Bands | H2O | 26.5 | |
| Bands | KBr | ||
| Bands | KBr | 3008 – 430 cm**(-1) | |
| Spectrum | KBr | 5000 – 667 cm**(-1) | |
| Bands | Grundschwingungsfrequenzen sowie Kraftkonstanten von Mol.schwing.. |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) |
| Spectrum | H2O |
| Spectrum | aq. HCl (20percent) |
| Spectrum | ethanol |
| Description (NQR Spectroscopy) | Nucleus (NQR Spectroscopy) |
| Nuclear quadrupole resonance | |
| Nuclear quadrupole coupling constants | 35Cl |
| Description (Luminescence Spectroscopy) |
| X-ray emission spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
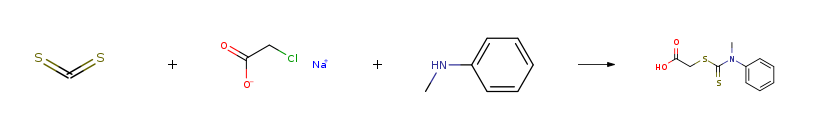
| Stage #1: carbon disulfide; N-methylaniline With sodium hydroxide In water at 20℃; for 4h; Stage #2: sodium monochloroacetic acid In water at 20℃; for 24h; Experimental Procedure Preparation of carboxymethyl-N-methyl-N-phenyl dithiocarbamate(DTC) A mixture of carbon disulfide (12 mL, 0.20 mol) and N-methylaniline (21.6 mL, 0.20 mol) was treated with sodium hydroxide (8.2 g, 0.20 mol) dissolved in 250 mL water. The organic layer disappears after stirring the solution for 4 h at room temperature. At this point the resulting straw coloured solution was treated with sodium choloroacetate (23.2 g, 0.20 mol) and allowed to stand for 24 hours. The resulting solution was acidified with conc. HCl and the solid which was separated, collected and dried. The purity of the ligand was checked by thin layer chromatographic technique. | 81% |
| With sodium hydroxide 1) 4h, RT, 2) 17h; Yield given. Multistep reaction; | |
| Stage #1: carbon disulfide; N-methylaniline With sodium hydroxide In water at 20℃; for 4h; Stage #2: sodium monochloroacetic acid In water at 20℃; for 17h; | 39.7g |
| With hydrogenchloride In water | |
| Stage #1: carbon disulfide; N-methylaniline With sodium hydroxide for 4h; Stage #2: sodium monochloroacetic acid at 20℃; Stage #3: With hydrogenchloride In water | |
| Stage #1: carbon disulfide; sodium monochloroacetic acid; N-methylaniline Stage #2: With sodium hydroxide Stage #3: With hydrogenchloride |
Safety and Hazards
| Pictogram(s) |    |
| Signal | Danger |
| GHS Hazard Statements | H301: Toxic if swallowed [Danger Acute toxicity, oral] H315: Causes skin irritation [Warning Skin corrosion/irritation] H400: Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard][Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P264, P270, P273, P280, P301+P310, P302+P352, P321, P330, P332+P313, P362, P391, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | UN 2659 6.1 / PGIII |
| Under the room temperature and away from light | |
| HS Code | 291540 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 116.479 |
| logP | -0.324 |
| HBA | 2 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 40.13 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (quant) | Unit | Effect |
| 4.67 | NOAEC | 2.5 – 10 | mg/L | Phytotoxic |
| 4.67 | NOAEC | 2.5 – 10 | mg/L | Phytotoxic |
| 4.59 | NOAEC | 3 – 20 | mg/L | Phytotoxic |
| 4.3 | EC50 | 5.8 – 9.7 | mg/L | Phytotoxic |
| 4.03 | EC50 | 10.8 – 23.5 | mg/L | Phytotoxic |
| 3.96 | EC25 | 4.3 – 7.6 | mg/L | Phytotoxic |
| 3.97 | EC25 | 6.3 – 20.9 | mg/L | Phytotoxic |
| EC10 | 2.7 – 19.8 | mg/L | Phytotoxic | |
| EC10 | 2.4 – 7 | mg/L | Phytotoxic |
| Quantitative Results | ||
| 1 of 7 | Effect | Cytotoxic |
| Assay Description | Effect : lactate release Target : Chang epithelial cells from human liver Bioassay : LDH: lactate dehydrogenase; lactate release as index of cytotoxicity; NADH formation was assumed to be proportional to amount of lactate present in the sample in vitro; proliferating confluent epithelial cells from the human liver (n=3); cells incub. with title comp. for 24 h in presence of NAD+ and LDH in hydrasine hydrate-glycine buffer (pH 9.4); formation of NADH recorded spectrophotometrically | |
| Results | lactate release into the culture medium: 25.5 μmol/1E6 cells; no increase in lactate release with increasing conc. up to 25 μg/ml, slight increase at higher conc. with a maximum at 50 μg/ml; graphic representation | |
| 2 of 7 | Effect | Cytotoxic |
| Assay Description | Effect : pyruvate release Target : Chang epithelial cells from human liver Bioassay : LDH: lactate dehydrogenase; pyruvate release as index of cytotoxicity; NADH disappearance was assumed to be proportional to amount of pyruvate present in the sample in vitro; proliferating confluent epithelial cells from human liver (n=3); cells incub. with title comp. for 24 h in presence of NADH and LDH in triethanolamine buffer (pH 7.6); pyruvate release recorded by NADH disappearance; spectrophotometry | |
| Results | pyruvate release into the culture medium: 646 nmol/1E6 cells; no increase in pyruvate release at conc. up to 25 μg/ml; more than 2-fold increase at 50 μg/ml; graphic representation | |
| 3 of 7 | Effect | Cytotoxic |
| Biological material | opossum epithelium cell | |
| Assay Description | Effect : lactate release Bioassay : LDH: lactate dehydrogenase; lactate release as index of cytotoxicity; NADH formation was assumed to be proportional to amount of lactate present in the sample in vitro; proliferating confluent epithelial cells from the human liver (n=3); cells incub. with title comp. for 24 h in presence of NAD+ and LDH in hydrasine hydrate-glycine buffer (pH 9.4); formation of NADH recorded spectrophotometrically | |
| Results | lactate release into the culture medium: 7.9 μmol/1E6 cells; no sign. increase in lactate release at conc. up to 25 μg/ml, with a more than 5-fold increase at 50 μg/ml; graphic representation | |
| 4 of 7 | Effect | Cytotoxic |
| Biological material | opossum epithelium cell | |
| Assay Description | Effect : pyruvate release Bioassay : LDH: lactate dehydrogenase; pyruvate release as index of cytotoxicity; NADH disappearance was assumed to be proportional to amount of pyruvate present in the sample in vitro; proliferating confluent epithelial cells from human liver (n=3); cells incub. with title comp. for 24 h in presence of NADH and LDH in triethanolamine buffer (pH 7.6); pyruvate release recorded by NADH disappearance; spectrophotometry | |
| Results | pyruvate release into the culture medium: 646 nmol/1E6 cells; no increase in pyruvate release at conc. up to 25 μg/ml; ca. 6-fold increase at 50 μg/ml; graphic representation | |
| 5 of 7 | Effect | Fatty-acid amide hydrolase 1:Wild |
| Assay Description | Effect : cytoskeletal alterations Target : Chang epithelial cells from human liver Bioassay : PBS: phosphate-buffered saline in vitro; cells incub. for 24 h with title comp. in PBS (pH 7.4); visualization of cytoskeleton by epifluorescence microscopy; ultrastructural characterization by transmission electron microscopy | |
| 6 of 7 | Effect | Cytotoxic |
| Biological material | opossum epithelium cell | |
| Assay Description | Effect : cytoskeletal alterations Bioassay : PBS: phosphate-buffered saline in vitro; epithelial cells from the proximal tubule; cells incub. for 24 h with title comp. in PBS (pH 7.4); visualization of cytoskeleton by epifluorescence microscopy; ultrastructural characterization by transmission electron microscopy | |
| Results | cells exhibited cytoplasmic vacuolization, membraneo disruption and especially mitochondrial alterations at 25 and 50 μg/ml; cytoskeletal reorganization obs. at all conc. tested | |
| 7 of 7 | Results | inhibited the ATC racemase in Pseudomonas desmolitica AJ 11898 |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Effect |
| 3.67 | EC50 | 25 | mg/L | Cytotoxic | |
| 3.07 | EC50 | 100 | mg/L | Cytotoxic | |
| 3.07 | EC50 | 100 | mg/L | Cytotoxic | |
| 3.07 | EC50 | 100 | mg/L | Cytotoxic | |
| Activity(insecticidal activity) | ND |
| Use Pattern |
| Chloroacetic acid sodium salt CAS#: 3926-62-3 as acid donor in dye dispersions |
| Chloroacetic acid sodium salt CAS#: 3926-62-3 as anionic agent |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watsonnoke Scientific Ltd | https://www.watsonnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |