Chloroquine diphosphate CAS#: 50-63-5; ChemWhat Code: 1392450
Identification
| Product Name | Chloroquine diphosphate |
| IUPAC Name | 4-N-(7-chloroquinolin-4-yl)-1-N,1-N-diethylpentane-1,4-diamine;phosphoric acid |
| Molecular Structure | 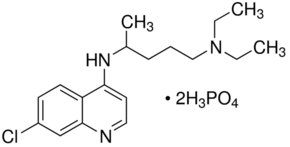 |
| CAS Registry Number | 50-63-5 |
| EINECS Number | 200-055-2 |
| MDL Number | MFCD00069852 |
| Beilstein Registry Number | 4223142 |
| Synonyms | chloroquine phosphate, Chloroquine phosphate, N4-(7-chloro-4-quinolinyl)-N1,N1-dimethyl-1,4-pentanediamine diphosphate, N4-(7-chloro-4-quinolinyl)-N1,N1-diethyl-1,4-pentanediamine diphosphate, N’-(7-chloroquinolin-4-yl)-N,N-diethyl-pentane-1,4-diamine diphosphate, chloroquine, diphosphate salt, chloroquine diphosphate salt CAS#: 50-63-5 CAS No. 50-63-5 |
| Molecular Formula | C18H26ClN3 · 2H3PO4 |
| Molecular Weight | 515.86 |
| InChI | InChI=1S/C18H26ClN3.2H3O4P/c1-4-22(5-2)12-6-7-14(3)21-17-10-11-20-18-13-15(19)8-9-16(17)18;21-5(2,3)4/h8-11,13-14H,4-7,12H2,1-3H3,(H,20,21);2(H3,1,2,3,4) |
| InChI Key | QKICWELGRMTQCR-UHFFFAOYSA-N |
| Canonical SMILES | CCN(CC)CCCC(C)Nc1ccnc2c1ccc(c2)Cl.OP(=O)(O)O.OP(=O)(O)O |
| Patent Information |
| No data available |
Physical Data
| Appearance | White Powder |
| Solubility | H2O: 50 mg/mL, clear |
| Melting Point, °C | Comment (Melting Point) |
| 193 – 200 | |
| 206.85 | |
| 176.85 | Method: slow heating. Crystallization with 1 Mol(s) H2O |
| 198.85 | Crystallization with 1 Mol(s) H2O |
| Description (Adsorption (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Adsorption isotherm | H2O | 37 | magnesium trisilicate |
| Adsorption isotherm | H2O | 37 | magnesium oxide |
| Adsorption isotherm | H2O | 37 | aluminium hydroxide |
| Adsorption isotherm | H2O | 37 | edible clay |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Comment (Association (MCS)) | Partner (Association (MCS)) |
| Association with compound | aq. buffer, dimethyl sulfoxide | 25 | ferriprotoporphyrin IX hydroxide | |
| Association with compound | aq. buffer | 37 | primary retinal pigment epithelial cells melanin granules | |
| Association with compound | aq. buffer, sodium chloride | linear pUC18 DNA containing 50% AT | ||
| Association with compound | various solvents | in the presence of salts | salmon testis DNA | |
| Formation constant of a complex | various solvent(s) | ambient temperature | α1-acid glycoprotein | |
| UV/VIS spectrum of the complex | various solvent(s) | in the presence of salts | salmon testis DNA | |
| Further physical properties of the complex | 37 | in the presence of additives | sodium salicylate |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Spectrum | 1H | water-d2 | 24.84 | 300 |
| 2D-NMR, Spectrum | 13C | D2O |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands, Spectrum | potassium bromide |
| Bands, Spectrum |
| Description (Mass Spectrometry) |
| liquid chromatography mass spectrometry (LCMS), electrospray ionisation (ESI), tandem mass spectrometry, spectrum |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | water | |||
| Spectrum | aq. buffer | |||
| Spectrum | aq. buffer, sodium chloride | 330, 342 | 14200, 14750 | |
| methanol | 330 | 19700 | ||
| toluene | 342 | 1700 | ||
| in the presence of additive(s), Spectrum | water, sulfuric acid | 343 | ||
| Spectrum | various solvents | Remark: pH 7.0 | ||
| aq. phosphate buffer | Remark: pH 7.2 | 342, 329, 255 | 19000, 18000, 16000 | |
| H2O | 342, 329, 255 | 20000, 18000, 17000 | ||
| D2O | Remark: pD 7, phosphate buffer | 343 | ||
| Absorption maxima | H2O | 329, 343 | 17000, 18000 |
Route of Synthesis (ROS)
| Conditions | Yield |
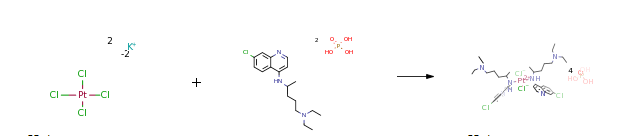
| In water at 20℃; for 18h; Experimental Procedure Synthesis of the trans-Pt(CQDP)2(Cl)2 Complex19) A solutionof K2[PtCl4] (100 mg, 0.24 mmol) in water (30 mL) wasstirred until it completely dissolved and then CQDP (250 mg,0.48 mmol) was added. Stirring was continued for 18 h at roomtemperature and a pink precipitate was obtained. This precipitatewas collected by filtration, washed with water, and driedunder vacuum.Yield 87.8%; elemental analysis (%) Calcd forC36H64N6Cl4O16P4Pt (1297.65 g mol-1): C 33.3; N 6.5; H 4.9.Found: C 31.7; N 6.7; H 4.4. IR ν(N-H) 3305 cm-1; ν(C=C)1616 cm-1; ν(C=N) 1581 cm-1; ν(Pt-Cl) 341 cm-1; ν(Pt-N)420 cm-1. UV-Vis 238 and 344 nm. 1H-NMR (DMSO-d6; δppm): 9.05 (1H, d, J=6.09 Hz, NH), 8.82 (1H, d, J=9.13 Hz,H5), 8.58 (1H, d, J=7.01 Hz, H2), 8.02 (1H, d, J=1.83 Hz, H8),7.8 (1H, dd, J1=1.83, J2=7.01 Hz, H6), 7.02 (1H, d, J=7.01 Hz,H3), 4.17 (1H, m, H1), 3.15 (6H, m, H4, H5), 1.79 (4H, m,H2, H3), 1.3 (3H, d, J=6.39 Hz, H1), 1.18 (6H, t, H6). | 87.8% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H302 (98.67%): Harmful if swallowed [Warning Acute toxicity, oral] H360 (32%): May damage fertility or the unborn child [Danger Reproductive toxicity] H373 (32%): Causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P201, P202, P260, P264, P270, P281, P301+P312, P308+P313, P314, P330, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) For more detailed information, please visit ECHA C&L website |
| Source: European Chemicals Agency (ECHA) License Note: Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: “Source: European Chemicals Agency, http://echa.europa.eu/”. Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. License URL: https://echa.europa.eu/web/guest/legal-notice Record Name: (1-Cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate URL: https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/213446 Description: The information provided here is aggregated from the “Notified classification and labelling” from ECHA’s C&L Inventory. Read more: https://echa.europa.eu/information-on-chemicals/cl-inventory-database |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | 293349 |
| Storage | Under the room temperature and away from light |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 515.868 |
| HBA | 7 |
| HBD | 4 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 115.73 |
| Rotatable Bond (RotB) | 8 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit |
| 10.7 | IC50 | 1.86E-05 | µM | |
| 8.88 | IC90 | 11.8 – 22.3 | nM | |
| 8.86 | IC90(Growth inhibition) | = | 19.92 | nM |
| 8.4 | percentage decrease(in hemozoin) | Active | ||
| 7.86 | IC50(Parasite Growth) | = | 13.8 | nM |
| 7.8 | percentage decrease(in hemozoin) | Active | ||
| 7.33 | IC50 | = | 4.7E-08 | M |
| 6.09 | IC90 | 912 | nm |
| Quantitative Results | ||
| 1 of 10 | Effect | Antiinflammatory agent |
| 2 of 10 | Effect | antiparasitic agent |
| 3 of 10 | Effect | antimalarial agent |
| 4 of 10 | Effect | antibabesial agent |
| Biological material | bovine erythrocyte | |
| 5 of 10 | Assay Description | Effect : Nrf2 activity; inhibition of Target : lung adenocarcinoma cells transfected with ARE-luciferase reporter vector Bioassay : Example 2 A Novel Assay for Nrf2 Inhibitors A high throughput approach to screen compounds was developed. A cell based reporter assay was used to identify agents that can inhibit Nrf2 mediated transcription. Lung adenocarcinoma cells that are stably transfected with ARE-luciferase reporter vector were |
| Results | title compound treatment showed inhibited luciferase activity of 47% | |
| 6 of 10 | Effect | Cytotoxic |
| Assay Description | Target : STHdhQ111 cells derived from Huntington’s disease (HD) knock-in mouse model of mouse Bioassay : Example 1: Screening Assays Provided are screening methods for identifying candidate compounds that treat, prevent, or ameliorate neurodegenerative disorders, e.g., HD.A variety of model systems, including cellular as well as animal models, have demonstrated that the exon 1 portion of Htt, containing | |
| 7 of 10 | Effect | antitrypanosomal agent |
| Biological material | trypanosoma brucei brucei cmp | |
| Assay Description | In vivo mean survival time of mouse (n = 6/batch) infected with TRYPANOSOMA BRUCEI BRUCEI CMP upon intraperitoneal administration of compound at a dose of 100 umol/kg | |
| 8 of 10 | Effect | inhibitory activity |
| Target | ||
| Substance action on target | ||
| Assay Description | HL-60 cell line | |
| Measurement | IC50 | |
| 9 of 10 | Assay Description | Equilibrium constant (stability) of the compound at pH 7.4 |
| Measurement | log Keq | |
| 10 of 10 | Biological material | Plasmodium falciparum |
| Assay Description | Vacuolar accumulation ratio of compound in inside and outside of the Plasmodium falciparum food vacuole was measured | |
| Results | Vacuolar accumulation ratio not calculated | |
| Measurement | Vacuolar accumulation ratio |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| pX | Parameter | Value (quant) | Unit |
| 8.33 | IC90 | 41.7 | nM |
| 8 | IC50 | 0.01 | µM |
| 7.8 | IC50 | 8.2 | ng/mL |
| 7.54 | MIC | 15 | ng/mL |
| 6.96 | IC50(Parasitized cells) | 0.11 | nM |
| 5.41 | IC50 | 2000 | ng/mL |
| 5.3 | inhibition rate(Colony formation) | ||
| 5 | percentage decrease | ||
| 4.7 | percentage decrease(DNA synthesis (EdU staining)) |
| Use Pattern |
| Chloroquine diphosphate CAS#: 50-63-5 as Pharmaceuticals |
| Chloroquine diphosphate CAS#: 50-63-5 as treating malaria in combination with trizolopyrimidinone |
| enhancing immunity |
| Chloroquine diphosphate CAS#: 50-63-5 as Refsum disease |
| Zellweger syndrome |
| cerebrohepatorenal syndrome |
| treating colon cancer in combination with an arginine degradation enzyme and a chemotherapy drug |
| Hedgehog antagonist |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |