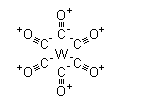Chromium hexacarbonyl CAS#: 13007-92-6; ChemWhat Code: 36712
Identification
| Product Name | Chromium hexacarbonyl |
| IUPAC Name | carbon monoxide;chromium |
| Molecular Structure | 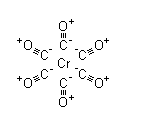 |
| CAS Registry Number | 13007-92-6 |
| EINECS Number | 235-852-4 |
| MDL Number | MFCD00010945 |
| Beilstein Registry Number | No data available |
| Synonyms | chromium(0) hexacarbonyl, hexa-carbonylchromate(0), hexacarbonyl chromium(0), hexacarbonylchromate(0), hexacarbonylchromium(0), hexacarbonylchromium(O), chromium hexacarbonyl; CAS Number: 13007-92-6;CAS No.:13007-92-6 |
| Molecular Formula | C6CrO6 |
| Molecular Weight | 220.057 |
| InChI | InChI=1S/6CO.Cr/c6*1-2; |
| InChI Key | KOTQLLUQLXWWDK-UHFFFAOYSA-N |
| Canonical SMILES | [C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[Cr] |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2008/77911 | LIGANDS AND CATALYST SYSTEMS FOR THE OLIGOMERIZATION OF OLEFINIC MONOMERS | 2008 |
Physical Data
| Appearance | White crystal |
| Solubility | insoluble |
| Refractive index | 1.5560 (estimate) |
| Melting Point, °C | Comment (Melting Point) |
| 130 | with decomposition |
| 149 – 150 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 147.4 | 760 |
| Density, g·cm-3 | Measurement Temperature, °C | Type (Density) |
| -173.16 | crystallographic | |
| 1.865 | -173.16 | crystallographic |
| 1.768 | crystallographic | |
| 1.766 – 1.772 | crystallographic | |
| 1.77 | crystallographic | |
| 1.77 | 18 |
| Description (Association (MCS)) | Comment (Adsorption (MCS)) | Partner (Association (MCS)) |
| Adsorption | sorption diagram | α-Fe2O3(0001) |
| MCM-41 | ||
| Adsorption | sorption diagram | silica |
| Adsorption | Pd | |
| silica-alumina | ||
| Adsorption | silica | |
| Adsorption | Cu(100) |
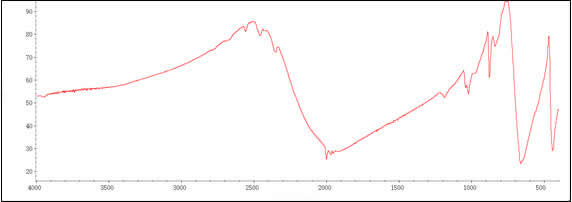
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C |
| 13C | chloroform-d1 | 80 | |
| 13C | CD2Cl2 | -63.16 | |
| Spectrum | 13C | C6D5CD3=toluene-d8 | -43.15 |
| Linewidth of NMR absorption | 13C | CDCl3 | 21 |
| Linewidth of NMR absorption | 17O | CDCl3 | 38 |
| 13C | methylene chloride=methylene dichloride | 22 | |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C | Signals, cm-1 |
| Intensity of IR bands, Bands | potassium bromide | ||
| Spectrum | cyclohexane | 25 | |
| Bands | hexane | ||
| Bands | tetrahydrofuran | 1983 | |
| Bands | gaseous matrix | -261.16 | 1984 |
| Bands | KBr | 1999 | |
| Spectrum | further solvent(s) | ||
| Spectrum | gaseous matrix | -261.16 |
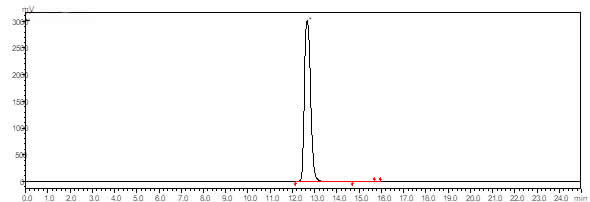
| Description (Mass Spectrometry) | Comment (Mass Spectrometry) |
| Spectrum | Fragmentation pattern |
| Spectrum | Molecular peak, Fragmentation pattern |
| Molecular peak | |
| Molecular peak, Fragmentation pattern | |
| mass spectrometry |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| Spectrum, Band assignment | gas | 200 nm – 400 nm | |
| Spectrum, Band assignment | neat (no solvent, gas phase) | 200 nm – 400 nm | |
| Spectrum | CHCl3 | 220 nm – 500 nm | 258 |
| Band assignment | acetonitrile | 2.88 eV – 5.11 eV | |
| 250 nm – 355 nm | |||
| Spectrum | hexane | 200 nm – 500 nm | |
| Band assignment | cyclohexane | 280 |
| Description (Raman Spectroscopy) |
| Bands |
| Raman |
Route of Synthesis (ROS)
| Conditions | Yield |
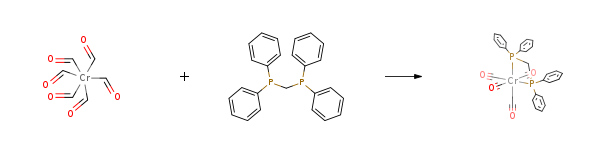
| In diethylene glycol mixt. Cr(CO)6 and bis(diphenylphosphino)methane in diglyme was heated at 135°C for 3.5 h; | 82% |
| in boiling solvent or in bomb tube at 140-190°C;; | 77% |
| In diethylene glycol other Radiation; mixt. Cr(CO)6 and 10 % excess bis(diphenylphosphino)methane was suspended in diglyme and under microwave irradiation was heated at 180°Cfor 5 min; react. mixt. was cooled to room temp., methanol was added, ppt. was collected by filtration and dried in vacuo; elem. anal.; | 55% |
| With sodium tetrahydroborate In butan-1-ol at 105℃; under 760.051 Torr; for 0.333333h; Microwave irradiation; Inert atmosphere; Green chemistry; | 54% |
| In tetrahydrofuran Irradiation (UV/VIS); (Ar); photolysis of a soln. of chromium complex and ligand in THF for 45min, stirring for 2 h; concn., column chromy. (silica gel, CH2Cl2/hexanes 1:10); | 26% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H301 (93.48%): Toxic if swallowed [Danger Acute toxicity, oral] H331 (10.87%): Toxic if inhaled [Danger Acute toxicity, inhalation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P270, P271, P301+P310, P304+P340, P311, P321, P330, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 6.1; Packaging Group: III; UN Number:3466 |
| Under the room temperature and away from light | |
| HS Code | 293190 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD 4500/kg |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 220.058 |
| logP | 0.402 |
| HBA | 6 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 102.42 |
| Rotatable Bond (RotB) | 6 |
| Matching Veber Rules | 2 |
| Laboratory Use and Handling | Use Pattern |
| Preparation of adducts with carbon nanotubes | |
| dissotiates under modulated MIR pulses | |
| information on use | |
| stable in weakly basic media | |
| undergoes thermal decompn. at 503-613 K | |
| in solution sensitive to light | |
| stable only in the dark; ppt. of brown flakes in diffuse light; | |
| decomposition with fuming nitric acid and indifferent against cold KOH, mineral acids, Br2, I2 |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |