cyclopropanol CAS#: 16545-68-9; ChemWhat Code: 161716
Identification
| Product Name | cyclopropanol |
| IUPAC Name | cyclopropanol |
| Molecular Structure | |
| CAS Registry Number | 16545-68-9 |
| MDL Number | MFCD19707103 |
| Synonyms | Cyclopropanol 16545-68-9 Hydroxycyclopropane Cyclopropyl alcohol MFCD19707103 TK7N9F9R3Y UNII-TK7N9F9R3Y C3H6O DTXSID40167949 CHEBI:188444 YOXHCYXIAVIFCZ-UHFFFAOYSA-N AMY35209 AKOS022183551 SB40661 SY028267 DB-050527 CS-0059134 EN300-100685 A858296 Q3560539 |
| Molecular Formula | C3H6O |
| Molecular Weight | 58.08 |
| InChI | InChI=1S/C3H6O/c4-3-1-2-3/h3-4H,1-2H2 |
| InChI Key | YOXHCYXIAVIFCZ-UHFFFAOYSA-N |
| Isomeric SMILES | C1CC1O |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN117486866 | Pyrimidinedione compound as well as preparation method and application thereof | 2024 |
| WO2023/205226 | KIT INHIBITORS | 2023 |
| CN115340514 | Chiral amine compound and preparation method thereof | 2022 |
| WO2019/25467 | SELECTIVE INHIBITORS OF NLRP3 INFLAMMASOME | 2019 |
| CN109912504 | Quinoline carboxylic acid compound and preparation method and application thereof | 2019 |
| CN106278887 | Synthesis method of 2,3,3,3-tetrafluoropropionate | 2017 |
| EP3190116 | PYRAZOLOTHIAZOLE COMPOUND AND MEDICINE | 2017 |
Physical Data
| Appearance | LiLight yellow liquid |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 101 | |
| 101 | |
| 52 | 90 |
| 101 | 760 |
| 100 – 102 | 760 |
| 100.5 – 102 | |
| 55.8 | 100 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 0.917 | 4 | 25 |
| 0.911 | 20 | 20 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Spectrum | 1H | CD3OD | |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | |
| Chemical shifts | 1H | chloroform-d1 | 500 |
| Chemical shifts | 1H | chloroform-d1 | 500 |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Spectrum | gas |
| Spectrum | Cneat (no solvent) |
| Bands | CCl4 |
| Bands |
Route of Synthesis (ROS)
| Conditions | Yield |
| With potassium tert-butylate In tetrahydrofuran at 80℃; for 3h; Experimental Procedure 1.1 Step 1 A mixture of El (1 g, 5.68 mmol), cyclopropanol (396 mg, 6.82 mmol) and KOtBu(2.1 g, 14.3 mmol) in THF (20 mL) was heated to 80°C for 3 hours. The mixture was added H2O (40 mL), extracted with EtOAc (40 mL x 3), and then the organic layer was washed with brine, dried over anhydrous Na2SC>4, filtered and concentrated in vacuo. The residue was purified by column chromatography (petroleum ether: EtOAc=4:l) to give the compound E2 (1.1 g, 90.4%) as a yellow oil. | 90.4% |
| With potassium tert-butylate In tetrahydrofuran at 80℃; for 3h; Experimental Procedure 1.1 Step 1 A mixture of El (1 g, 5.68 mmol), cyclopropanol (396 mg, 6.82 mmol) and KOtBu(2.1 g, 14.3 mmol) in THF (20 mL) was heated to 80°C for 3 hours. The mixture was added H2O (40 mL), extracted with EtOAc (40 mL x 3), and then the organic layer was washed with brine, dried over anhydrous Na2SC>4, filtered and concentrated in vacuo. The residue was purified by column chromatography (petroleum ether: EtOAc=4:l) to give the compound E2 (1.1 g, 90.4%) as a yellow oil. | 90.4% |
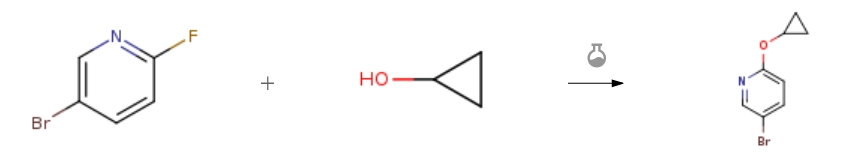
| With potassium tert-butylate In tetrahydrofuran; 1-methyl-pyrrolidin-2-one at 0 – 20℃; for 2h; Experimental Procedure 1 Step 1: 5-Bromo-2-cyclopropoxypyridine: To a stirred solution of 5- bromo-2-fluoropyridine (12.0 g, 68.6 mmol) in NMP (120 mL) was added cyclopropanol (5.97 g, 102.9 mmol), Potassium t-butoxide solution in THF (1M, 103 mL, 102.9 mmol) at 0 °C and stirred at rt for 2 h. The reaction mixture was quenched with cold water (0-5 °C) and extracted with 50% EtOAc in pet-ether (2 x 500 mL). The combined organic layer was washed with water and dried over sodium sulfate and concentrated under reduced pressure to afford 5-bromo-2-cyclocycloxypyridine (11.2 g, 76% yield) which was used directly in the next step. MS (ESI) m/z 213.8 [M+H]+. | 76% |
Safety and Hazards
| Pictogram(s) |   |
| Signal | Danger |
| GHS Hazard Statements | H301 (92.68%): Toxic if swallowed [Danger Acute toxicity, oral] H318 (92.68%): Causes serious eye damage [Danger Serious eye damage/eye irritation] |
| Precautionary Statement Codes | P264, P264+P265, P270, P280, P301+P316, P305+P354+P338, P317, P321, P330, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Store at 2°C ~ 8°C for long time, in container tightly sealed; Protect from light. |
| HS Code | |
| Storage | Store at 2°C ~ 8°C for long time, in container tightly sealed; Protect from light. |
| Shelf Life | 1 year |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 58.08 |
| logP | 0.044 |
| HBA | 1 |
| HBD | 1 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 20.23 |
| Rotatable Bond (RotB) | 0 |
| Matching Veber Rules | 2 |
| Use Pattern |
| Cyclopropanol is an organic compound with a three-carbon ring structure, and Cyclopropanol serves as an important intermediate in the synthesis of various organic compounds. Its ring structure imparts unique reactivity in chemical reactions, making it valuable for constructing more complex molecules. In pharmaceutical chemistry, cyclopropanol is often used as a key precursor for synthesizing active pharmaceutical ingredients (APIs). Its unique three-carbon ring can provide specific physicochemical properties and biological activities to drug molecules. Cyclopropanol is utilized in the synthesis of bioactive molecules such as pesticides and insecticides. Its small ring structure is significant in the design of molecules with specific biological activities. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |