D-Camphorsulfonic acid CAS#: 3144-16-9; ChemWhat Code: 82266
Identification
| Product Name | D-Camphorsulfonic acid |
| IUPAC Name | [(1S,4R)-7,7-dimethyl-2-oxo-1-bicyclo[2.2.1]heptanyl]methanesulfonic acid |
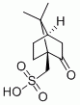
| Molecular Structure |  |
| CAS Registry Number | 3144-16-9 |
| EINECS Number | 221-554-1 |
| MDL Number | MFCD00064157 |
| Beilstein Registry Number | 2809675 |
| Synonyms | (1S)-10-camphorsulfonic acid, d-10-camphorsulfonic acid |
| Molecular Formula | C10H16O4S |
| Molecular Weight | 232.301 |
| InChI | InChI=1S/C10H16O4S/c1-9(2)7-3-4-10(9,8(11)5-7)6-15(12,13)14/h7H,3-6H2,1-2H3,(H,12,13,14)/t7-,10-/m1/s1 |
| InChI Key | MIOPJNTWMNEORI-GMSGAONNSA-N |
| Canonical SMILES | CC1(C2CCC1(C(=O)C2)CS(=O)(=O)O)C |
| Isomeric SMILES | CC1([C@@H]2CC[C@]1(C(=O)C2)CS(=O)(=O)O)C |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2018/170476 | POLYMORPHIC COMPOUNDS AND USES THEREOF | 2018 |
| CN103509029 | Phenanthroquinolizidine Phenanthroindolizidine alkaloid derivatives and salts thereof and their preparation, anti-plant-virus and anti-cancer activity (by machine translation) | 2016 |
| US2013/150360 | INHIBITORS OF BRUTON’S TYROSINE KINASE | 2013 |
Physical Data
| Appearance | Off white crystalline powder |
| Alpha | 22 º (589nm, c=20, H2O 25 ºC) |
| Boiling Point | 100 °C |
| Refractive index | 21.5 ° (C=5, H2O) |
| Solubility | Exhibit moderate Solubulity in HCCl. |
| PH | 0.3 (200g/l, H2O) |
| Stability | Stable. Protect from moisture. Incompatible with strong oxidizing agents, strong bases. |
| Sensitive | Hygroscopic |
| Water Solubility | Soluble in water |
| Melting Point, °C | Solvent (Melting Point) |
| 196 – 200 | |
| 194.9 – 195.8 | ethyl acetate |
| 195 | acetic acid |
| 197 – 198 | benzene |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Formation constant of a complex | aq. phosphate buffer | 24.99 | β-cyclodextrin |
| Association with compound | CDCl3 | -60 | Dimethyl-(2-methyl-8-phenyl-quinolin-5-yl)-amine |
| Association with compound | CDCl3 | -60 | [8-(6-Methoxy-naphthalen-2-yl)-2-methyl-quinolin-5-yl]-dimethyl-amine |
| Association with compound | CDCl3 | -60 | 5-(Dimethylamino)-8-(3,5-diphenylphenyl)-2-methylquinoline |
| Dissociation Exponent (pK) | Dissociation Group | Solvent (Dissociation Exponent) | Type (Dissociation Exponent) |
| 1.2 | SO2OH | dichloromethane | a1/apparent |
Spectra
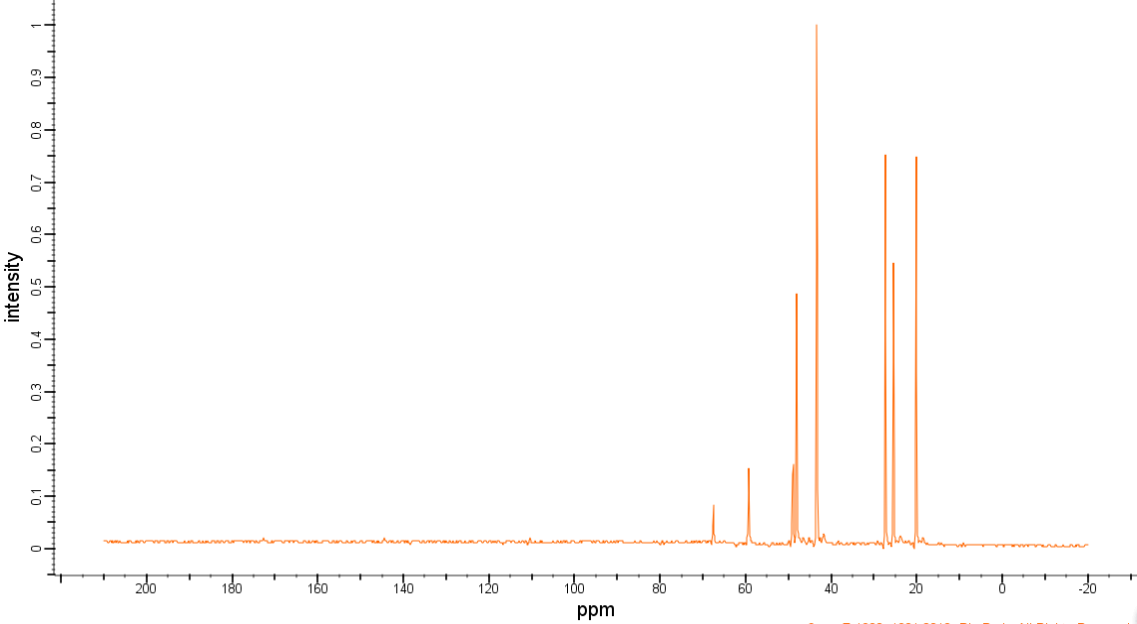
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | water-d2 | 300 | |
| Chemical shifts | 1H | [D3]acetonitrile | 400 | |
| Chemical shifts | 13C | CDCl3 | ||
| Chemical shifts | 33S | H2O | 37 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Spectrum | potassium bromide |
| D-Camphorsulfonic acid CAS#: 3144-16-9 IR1 | 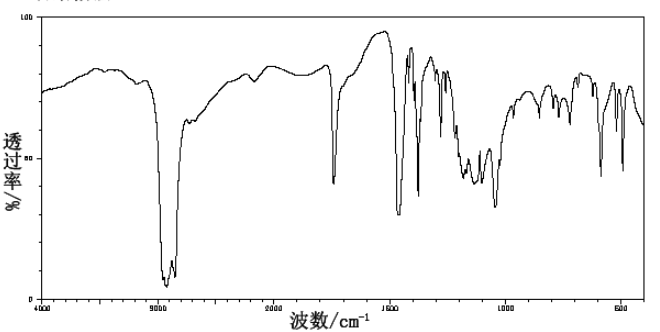 |
| D-Camphorsulfonic acid CAS#: 3144-16-9 IR2 | 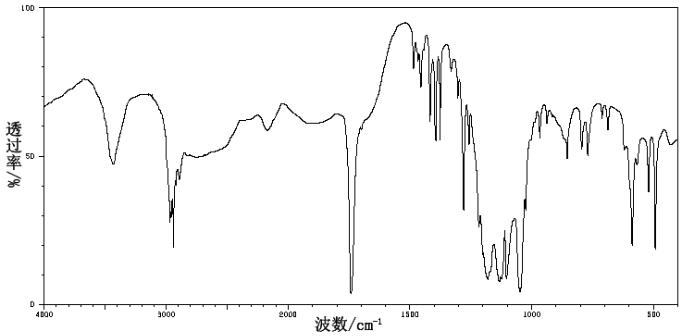 |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) |
| Spectrum | ethanol |
| Spectrum | H2O |
| D-Camphorsulfonic acid CAS#: 3144-16-9 MS | 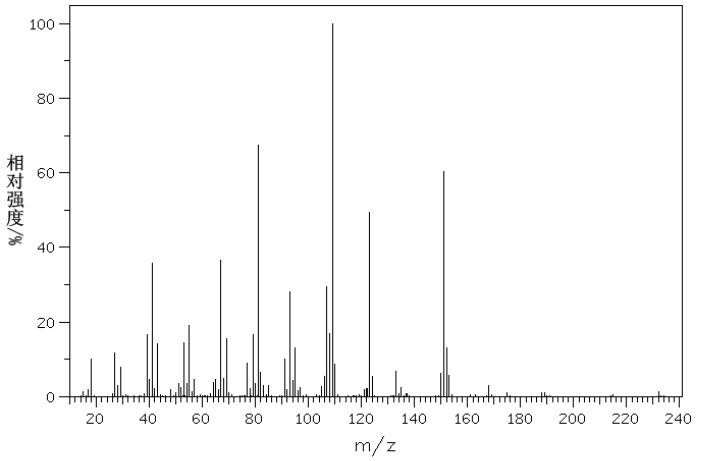 |
Route of Synthesis (ROS)
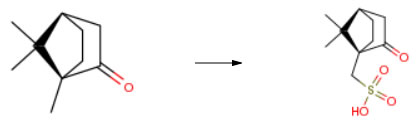
| Conditions | Yield |
| With sulfuric acid; acetic anhydride |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H290: May be corrosive to metals [Warning Corrosive to Metals] H314: Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] H318: Causes serious eye damage [Danger Serious eye damage/eye irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P234, P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P390, P404, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | UN number: 2585; Class: 8; Packing group: III |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD 60/kg |
| Use Pattern |
| (1S)-(+)-10-Camphorsulfonic acid may be used as a starting material to synthesize 10-iodocamphor. It may also be used as a dopant to induce chirality in the conduction band of polyaniline.(±)-S-methyl-S-phenylsulfoximine can be resolved using it as a chiral resolving agent to form the (-)-form in high enantiomeric purity. Used as a resolving agent, as a catalyst for coupling dipeptides. |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |