(Diacetoxyiodo)benzene CAS#: 3240-34-4; ChemWhat Code: 347305
Identification
| Product Name | (Diacetoxyiodo)benzene |
| IUPAC Name | [acetyloxy(phenyl)-λ3-iodanyl] acetate |
| Molecular Structure | 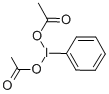 |
| CAS Registry Number | 3240-34-4 |
| EINECS Number | 221-808-1 |
| MDL Number | MFCD00008692 |
| Beilstein Registry Number | 1879369 |
| Synonyms | [bis(acetoxy)iodo]benzene, (diacetoxyiodo)benzene |
| Molecular Formula | C10H11IO4 |
| Molecular Weight | 322.099 |
| InChI | InChI=1S/C10H11IO4/c1-8(12)14-11(15-9(2)13)10-6-4-3-5-7-10/h3-7H,1-2H3 |
| InChI Key | ZBIKORITPGTTGI-UHFFFAOYSA-N |
| Canonical SMILES | CC(=O)OI(C1=CC=CC=C1)OC(=O)C |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2019/2487 | SYNTHESIS OF HYPERVALENT IODINE REAGENTS WITH DIOXYGEN | 2019 |
| US2012/53146 | PESTICIDAL COMPOSITIONS | 2012 |
| US2013/79324 | PYRROLOPYRIMIDINE AND PURINE DERIVATIVES | 2013 |
Physical Data
| Appearance | White to off white crystalline powder |
| Density | 1.815 |
| Refractive index | n/D 1.444 |
| Melting Point, °C | Solvent (Melting Point) |
| 161 – 163 | acetic acid |
| 146 – 148 | dichloromethane, Petroleum ether, diethyl ether |
| 155 – 157 | chloroform, hexane |
| 157 – 159 | benzene |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| NMR spectrum of the complex | CDCl3 | 20 | I2 |
| NMR spectrum of the complex | CDCl3 | 20 | C5H9IO, I2 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz | Original Text (NMR Spectroscopy) |
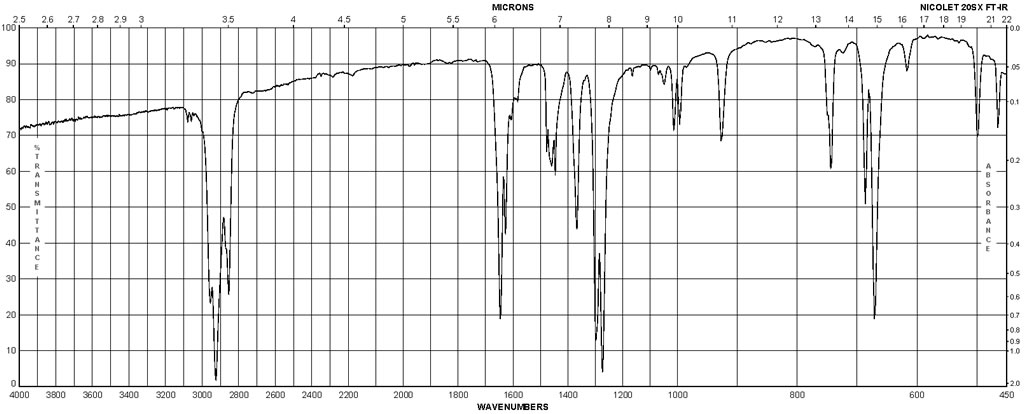
| Chemical shifts | 1H | chloroform-d1 | 23 | 1H NMR (δ, 23° C., CDCl3): 8.09 (d, J=7.3 Hz, 2H), 7.63-7.47 (m, 3H), 2.01 (s, 6H). | |
| Chemical shifts | 13C | chloroform-d1 | 23 | 13C NMR (δ, 23° C., CDCl3): 176.5, 135.0, 131.8, 131.0, 121.7, 20.5 | |
| Chemical shifts | 1H | chloroform-d1 | 24.84 | 400 | |
| Chemical shifts | 13C | chloroform-d1 | 24.84 | 100 |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) |
| Spectrum | methanol |
| Spectrum | acetonitrile |
| Spectrum | acetone, dichloromethane |
Route of Synthesis (ROS)
| Conditions | Yield |
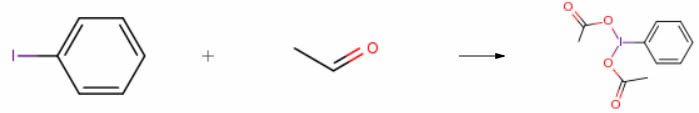
| With cobalt(II) chloride hexahydrate; oxygen; acetic acid at 23℃; under 760.051 Torr; for 5h; Reagent/catalyst; Sealed tube; Experimental Procedure General procedure: In a typical experiment, a 20 ml scintillation vial was charged with glacial AcOH (2 ml), iodoarene (0.401 mmol) andCoCl2·6H2O (0.004 mmol, 1 mol%) and was fitted with a rubber septum. The reaction vessel was purged with O2 for 5 min before acetaldehyde (4.07 mmol, 10.2 equiv.) was added in one portion. The reaction mixture was stirred under 1 atm O2, delivered by inflated balloon at 23 °C for 5 h. The solvent was removed in vacuo and the residuewasdissolved in CH2Cl2. The organic layer was washed with distilled water and extracted with CH2Cl2 (3 × 7 ml). The organic layer was dried over MgSO4 and solvent was removed in vacuo to afford the corresponding iodobenzene diacetate. The isolatedcompounds were characterized by 1H and 13C NMR spectroscopies. | 92% |
| With cobalt(II) chloride hexahydrate; oxygen In acetic acid at 23℃; under 760.051 Torr; for 5h; Solvent; Reagent/catalyst; Experimental Procedure A 20-mL scintillation vial was charged with 18 glacial AcOH (2 mL), 19 iodobenzene (82.2 mg, 0.401 mmol, 1.00 equiv) and 20 CoCl2.6H2O (0.9 mg, 0.004 mmol, 1 mol %) and was fitted with a rubber septum. The reaction vessel was purged with O2 for 5 min before 21 acetaldehyde (224 μL, 4.07 mmol, 10.2 equiv) was added in one portion. The reaction mixture was stirred under 1 atm O2, delivered by inflated balloon, at 23° C. for 5 h. The solvent was removed in vacuo and residue was dissolved in 22 CH2Cl2. The organic layer was washed with distilled water and extracted with CH2Cl2 (3×7 mL). The organic layer was dried over MgSO4 and solvent was removed in vacuo to afford 119 mg of 23 iodobenzene diacetate (1a) as white solid (92% yield). Characterization of 1a: 1H NMR (δ, 23° C., CDCl3): 8.09 (d, J=7.3 Hz, 2H), 7.63-7.47 (m, 3H), 2.01 (s, 6H). 13C NMR (δ, 23° C., CDCl3): 176.5, 135.0, 131.8, 131.0, 121.7, 20.5. The obtained spectral data are in good agreement with those reported in literature. (0067) A 20-mL scintillation vial was charged with iodobenzene diacetate (1a) (97.1 mg, 0.301 mmol, 1.00 equiv) and 3 M NaOH (5 mL). The reaction mixture was stirred for 3 h at 23° C. The resulting suspension was then filtered to afford 62 mg of iodosylbenzene (2a) as yellow solid (93% yield). Characterization of 2a: 1H NMR (δ, 23° C., CD3OD): 8.04 (dd, J=7.5, 2.1 Hz, 2H), 7.60-7.56 (m, 3H). 13C NMR data have not been collected due to poor solubility of 2a. HRMS (ESI+): Calcd. for C6H6IO [M+H]+ m/z 220.9463. The recorded spectral data are in good agreement with those reported in literature | 92% |
| With cobalt(II) chloride hexahydrate; oxygen In 1,2-dichloro-ethane at 23℃; under 760.051 Torr; for 1h; Kinetics; Solvent; Reagent/catalyst; Experimental Procedure A 20-mL scintillation vial was charged with DCE (0.5 mL), iodobenzene (20.2 mg, 0.0990 mmol, 1.00 equiv),and CoCl2*6H2O (0.2 mg, 0.9 μmol, 1 mol%), and was fitted with a rubber septum. The reaction vessel was purged with O2 for 5 min before acetaldehyde (56 μL, 1.0 mmol, 10 equiv) was added in one portion.The reaction mixture was stirred under 1 atm O2, delivered by inflated balloon, at 23 °C for 1 h. PBN (24.3mg, 0.138 mmol, 1.39 equiv) was added to the reaction mixture and stirred for 5-10 min. The resulting solution was injected in 2 mm EPR tube and analyzed. The acquired EPR spectrum is pictured in FigureS3a. |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H302: Harmful if swallowed [Warning Acute toxicity, oral] H312: Harmful in contact with skin [Warning Acute toxicity, dermal] H315: Causes skin irritation [Warning Skin corrosion/irritation] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335: May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Use Pattern |
| (Diacetoxyiodo)benzene CAS#: 3240-34-4 is usoxidant for preparing a cyclic phosphinate derivative by an intramolecular carbon-oxygen coupling reaction on a phosphinic acid derivative |
| (Diacetoxyiodo)benzene CAS#: 3240-34-4 is used as a hypervalent iodine oxidant |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |