Diethyl fluoromalonate CAS#: 685-88-1; ChemWhat Code: 55146
Identification
| Product Name | Diethyl fluoromalonate |
| IUPAC Name | diethyl 2-fluoropropanedioate |
| Molecular Structure | 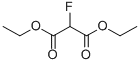 |
| CAS Registry Number | 685-88-1 |
| EINECS Number | 211-684-7 |
| MDL Number | MFCD00009139 |
| Beilstein Registry Number | 1775686 |
| Synonyms | Diethyl fluoromalonate 685-88-1 Diethyl 2-fluoromalonate Fluoromalonic acid diethyl ester 1,3-diethyl 2-fluoropropanedioate |
| Molecular Formula | C7H11FO4 |
| Molecular Weight | 178.16 |
| InChI | InChI=1S/C7H11FO4/c1-3-11-6(9)5(8)7(10)12-4-2/h5H,3-4H2,1-2H3 |
| InChI Key | GOWQBFVDZPZZFA-UHFFFAOYSA-N |
| Canonical SMILES | CCOC(=O)C(C(=O)OCC)F |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2014/148603 | Method for preparing a fluorinated organic compound | 2014 |
| US2013/90328 | COMPOUNDS FOR THE TREATMENT AND PROPHYLAXIS OF RESPIRATORY SYNCYTIAL VIRUS DISEASE | 2013 |
| EP2674430 | AMINO GROUP-CONTAINING PYRROLIDINONE DERIVATIVE | 2013 |
| EP2650276 | METHOD FOR PRODUCING -FLUOROALCOHOL | 2013 |
| WO2003/87064 | NOVEL PIPERAZINE DERIVATIVES AS INHIBITORS OF STEAROYL-COA DESATURASE | 2003 |
Physical Data
| Appearance | Colorless to pale yellow liquid |
| Flash Point | 62 °C |
| Refractive index | n20/D 1.407 (lit.) |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 102 – 103 | 13.5014 |
| 110 – 111 | 20 |
| 72 – 74 | 3.5 |
| 94 – 96 | 12 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.1475 | 20 | 20 |
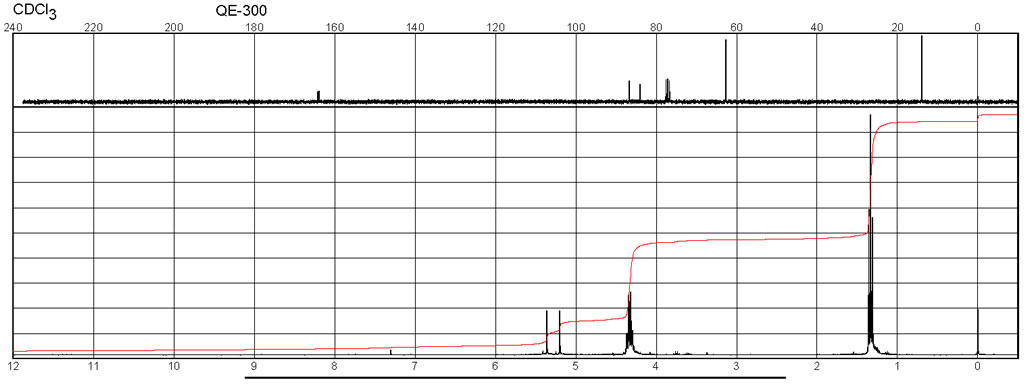
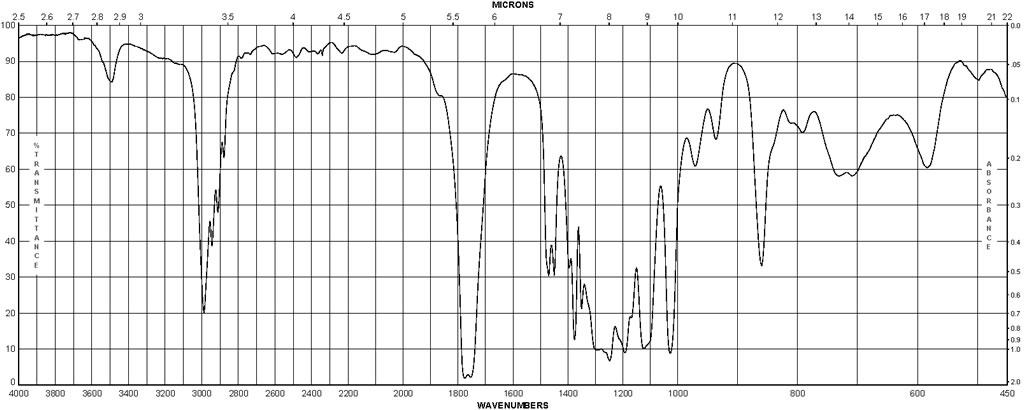
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 25 | 400 |
| Chemical shifts, Spectrum | 13C | chloroform-d1 | 25 | 101 |
| Chemical shifts, Spectrum | 19F | chloroform-d1 | 25 | 376 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Comment (IR Spectroscopy) |
| Bands | CCl4 | 2985 – 1032 cm**(-1) |
| Bands | nujol | 1745 – 1770 cm**(-1) |
Route of Synthesis (ROS)

| Conditions | Yield |
| With copper nitrate hemi(pentahydrate); fluorine In acetonitrile at 0 – 5℃; for 2h; Time; Temperature; Concentration; Green chemistry; | 94% |
| With triethylamine pentahydrogen fluoride salt; iodosylbenzene In 1,2-dichloro-ethane at 70℃; for 24h; Reagent/catalyst; Concentration; Experimental Procedure Fluorination of Malonic Esters 1; General Procedure General procedure: PhIO (550 mg, 2.5 mmol), Et3N·5HF (800 mg, 4 mmol), and DCE (1 mL)were placed in a Teflon test tube. After stirring at r.t. for 15 min, the appropriate malonic ester 1 (1 mmol) and DCE (1 mL) were added. The test tube was sealed with a septum rubber and heated at 70 °C for 24 h with stirring. The reaction mixture was neutralized with aq NaHCO3 and the product was extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were washed with brine (20 mL), dried (Na2SO4), and concentrated under reduced pressure. The product was purified by column chromatography on silica gel with hexane–CH2Cl2 as eluent. | 85% |
| With fluorine; copper(II) nitrate In acetonitrile at 5 – 8℃; for 4h; | 78% |
| With titanium(IV) isopropylate; N-fluorobis(methanesulfonyl)imide In dichloromethane at 20℃; for 12h; Reagent/catalyst; Solvent; Experimental Procedure 1 Preparation of diethyl fluoromalonate (3) Test tube equipped with a stir bar N- fluoro bis (methanesulfonyl) imide (382.4mg, 2.0mmol) and 1,2 g of dichloroethane (10 mL), at room temperature, and dissolved.Was then added the solution of diethyl malonate (160.2mg, 1.0mmol) and tetra -iso- propoxy orthotitanate (0.16 mL, 0.5 mmol), it was carried out at room temperature, 12 hours.After completion of the reaction, addition of water, dichloromethane extraction (3 times), the combined organic layers washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to give a crude product. The resulting crude product was used hexafluorobenzene as internal standard in 19F-NMR measurement, fluoro diethyl (3) of the desired product in 57percent yield was formed.Then purified by silica gel column chromatography (toluene / dichloromethane = 50/50 vol / vol) ,to obtain a purified fluoro diethyl colorless transparent liquid (89.0 mg, 0.50 mmol, 50percent yield). | 50% |
| With triethylamine pentahydrogen fluoride salt; iodosylbenzene In 1,2-dichloro-ethane at 70℃; for 24h; Reagent/catalyst; Experimental Procedure 1 Example 1 : Preparation of Diethyl Fluoromalonate (5) Into a 10 ml Teflon (registered trademark) test tube equipped with a stirring bar, Iodosylbenzene (550 mg, 2.50 mmol), triethylamine.hydrogen perfluoride (804 mg, 4.00 mmol) and 1,2-dichloroethane (2 ml) were added and stirred at room temperature for 15 minutes, then diethyl malonate(160 mg, 1.00 mmol) was added. Next, after sealing the test tube with a septum cap, it was heated to 70 ° C. on an oil bath and reacted for 24 hours.After completion of the reaction, the temperature was returned to room temperature, added to saturated aqueous sodium hydrogen carbonate solution (50 ml), neutralized and extracted three times with dichloromethane (10 ml), the organic layers were combined, washed with saturated brine (10 ml) After drying, filtration and concentration, a crude product was obtained. The obtained crude product was quantitatively determined by 1H-NMR measurement using 1,4-dimethoxybenzene as an internal standard substance, and the reaction yield was 100percent. Subsequently, the resulting crude product was purified by silica gel column chromatography to obtain diethyl fluoromalonate as a target product in a yield of 38percent.1H-NMR (400 MHz, CDCl3) delta 1.34 (t, Hz [ J= 7 ], 6H, CH3), 4.33 (q, J= 7 Hz, 4H, CH2), 5.28 (d, J= 48.5 Hz, 1H, CHF). 13C-NMR(100-MHz, CDCl3) delta13.9, 62.6-85.2 (d, J= 195 Hz), 163.9 (d, J= 24 Hz). 19F-NMR (376 MHz, CDCl3) delta-195.17 (d, J= 48.5 Hz). | 38% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H314: Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 8; Packaging Group: II; UN Number: 3265 |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD 45/kg |
| Use Pattern |
| Intermediate product for production of 4,6-dichloro-5-flouoro-pyrimidine. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |